Sources and control factors of main ions and dissolved inorganic carbon in karst water of the Huixian karst wetland, Guangxi
-
摘要:
研究目的 为查明会仙岩溶湿地水体主要离子和溶解无机碳(DIC)的来源及控制因素。
研究方法 于会仙岩溶湿地采集地下水和地表水样品,分析了水化学和溶解无机碳同位素(δ13CDIC)参数特征。
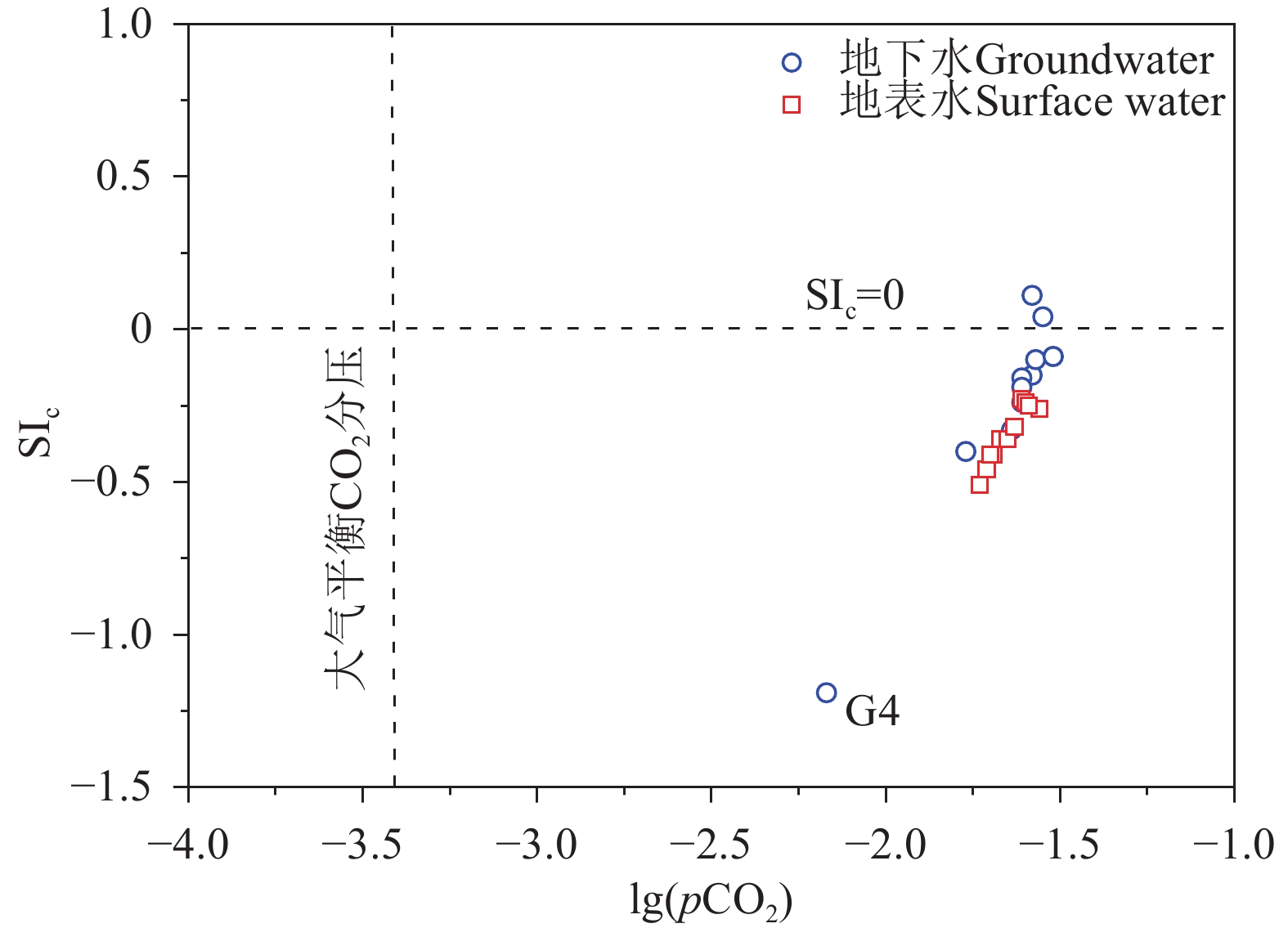
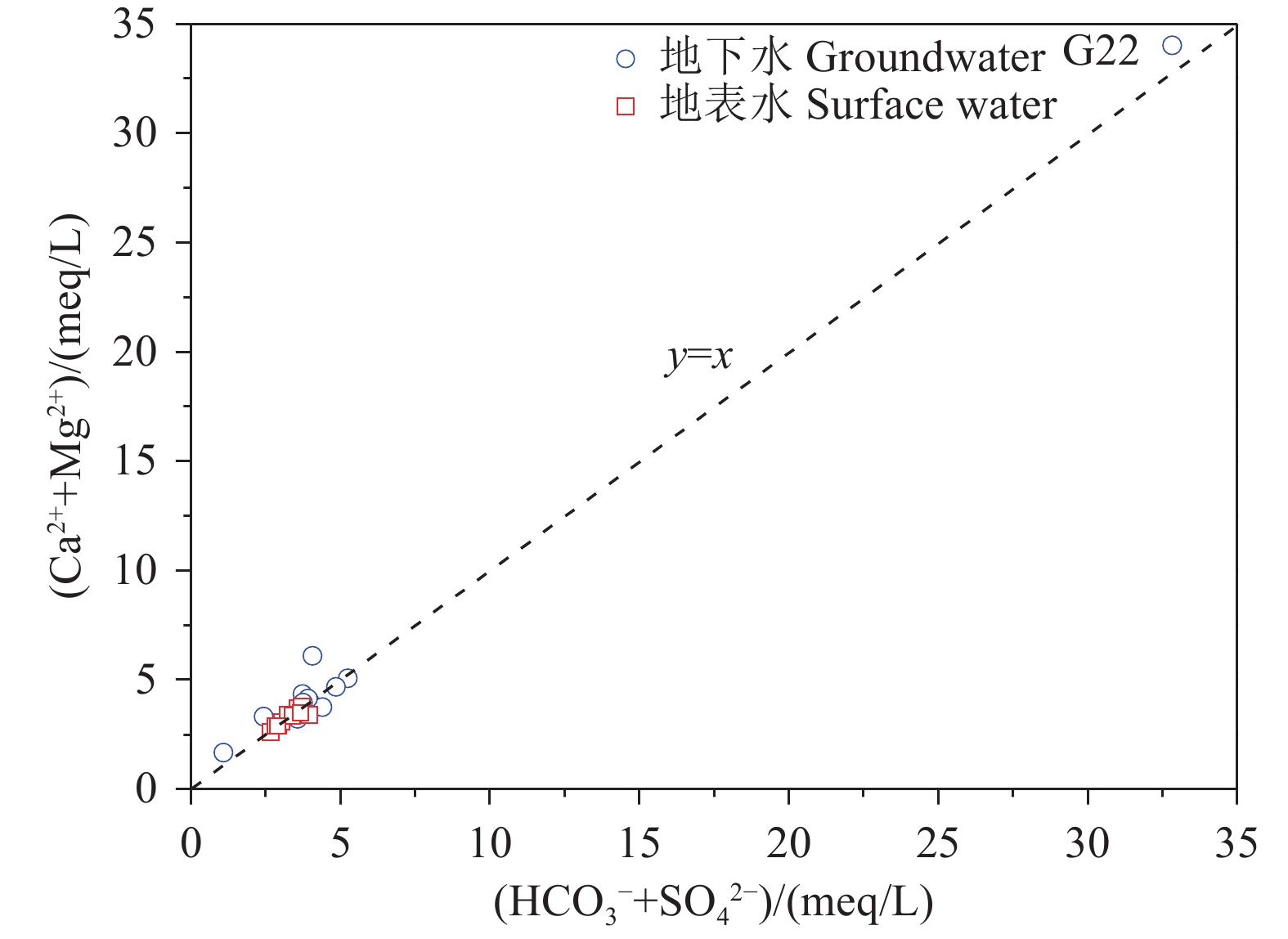
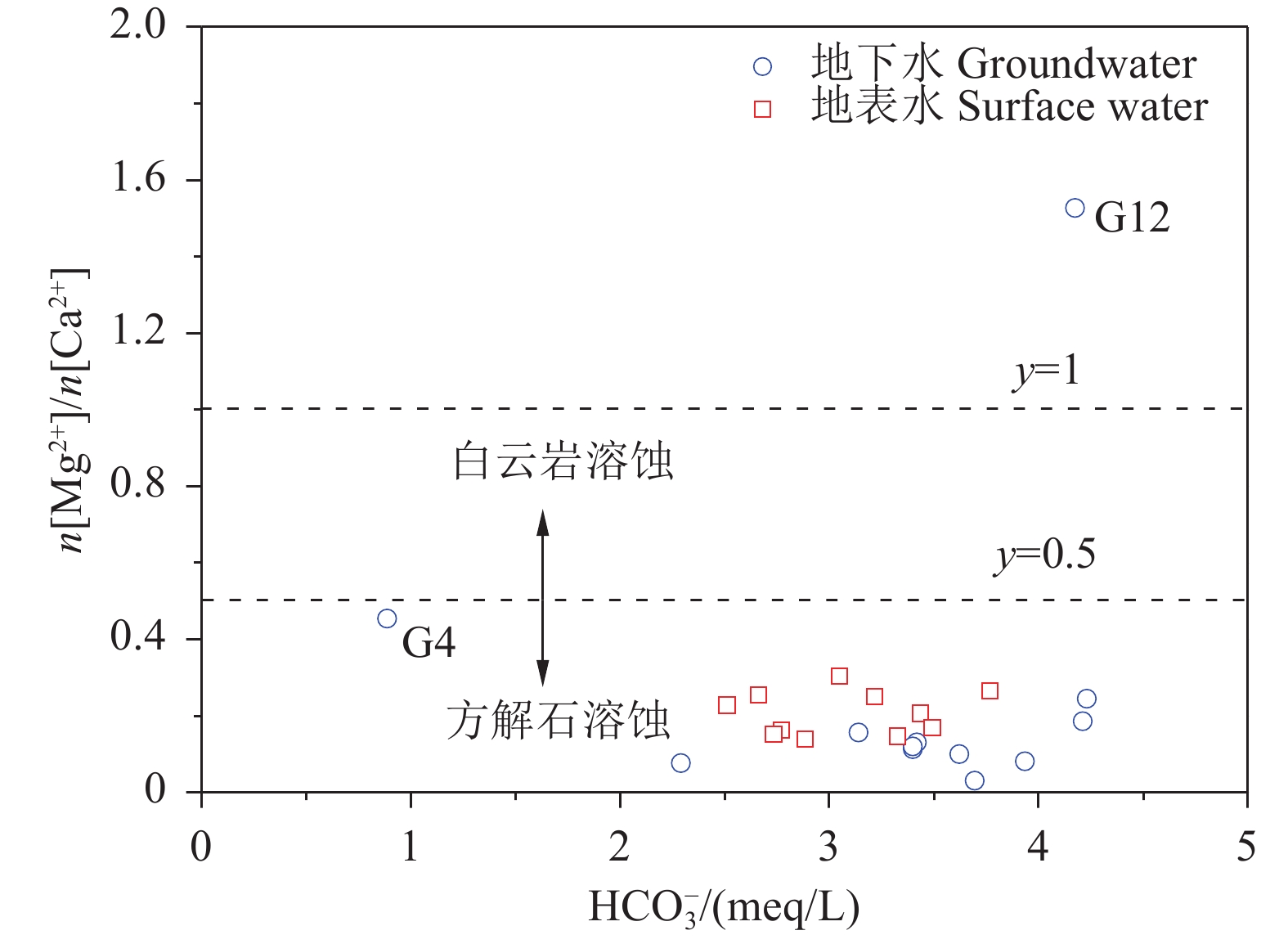
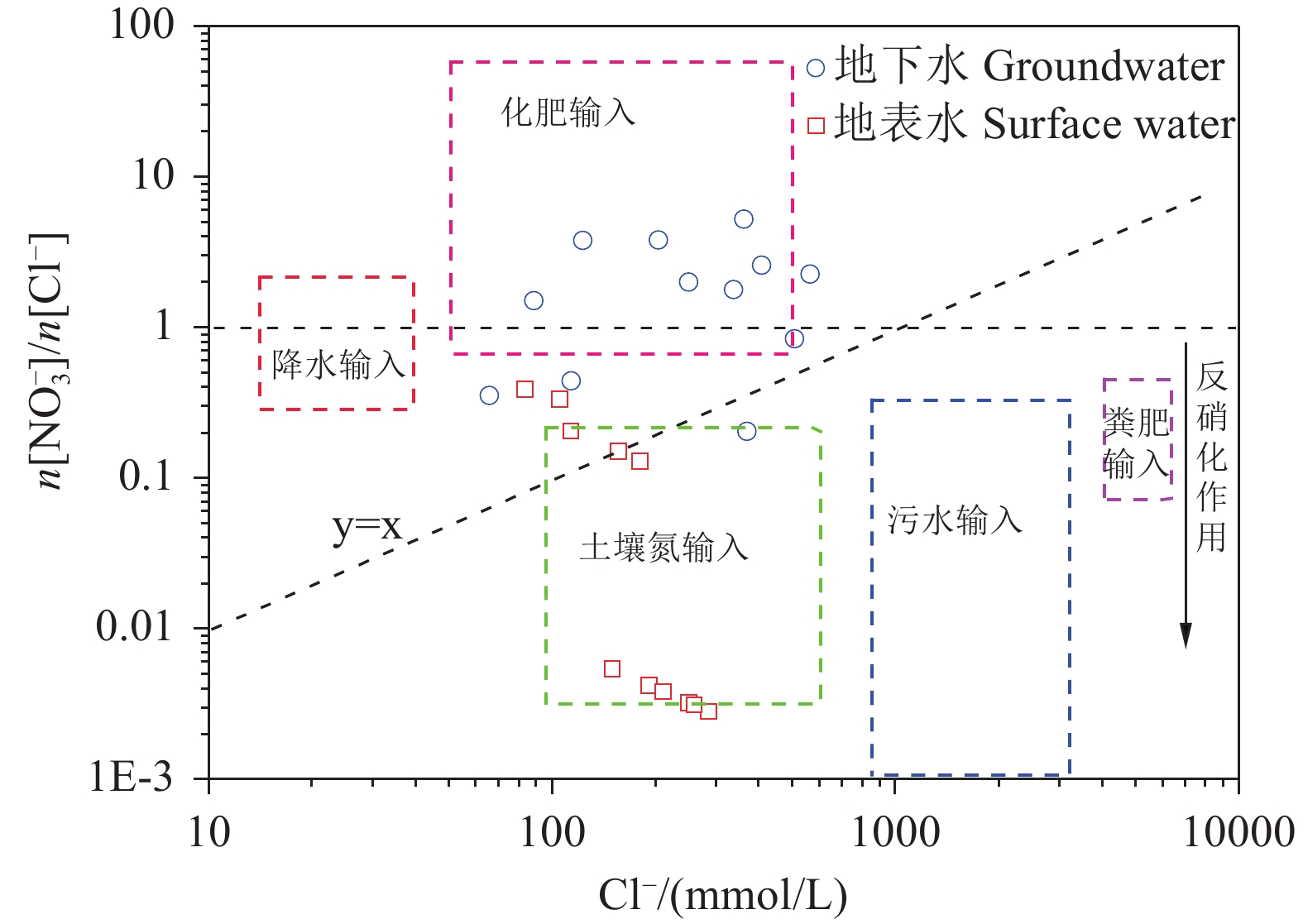
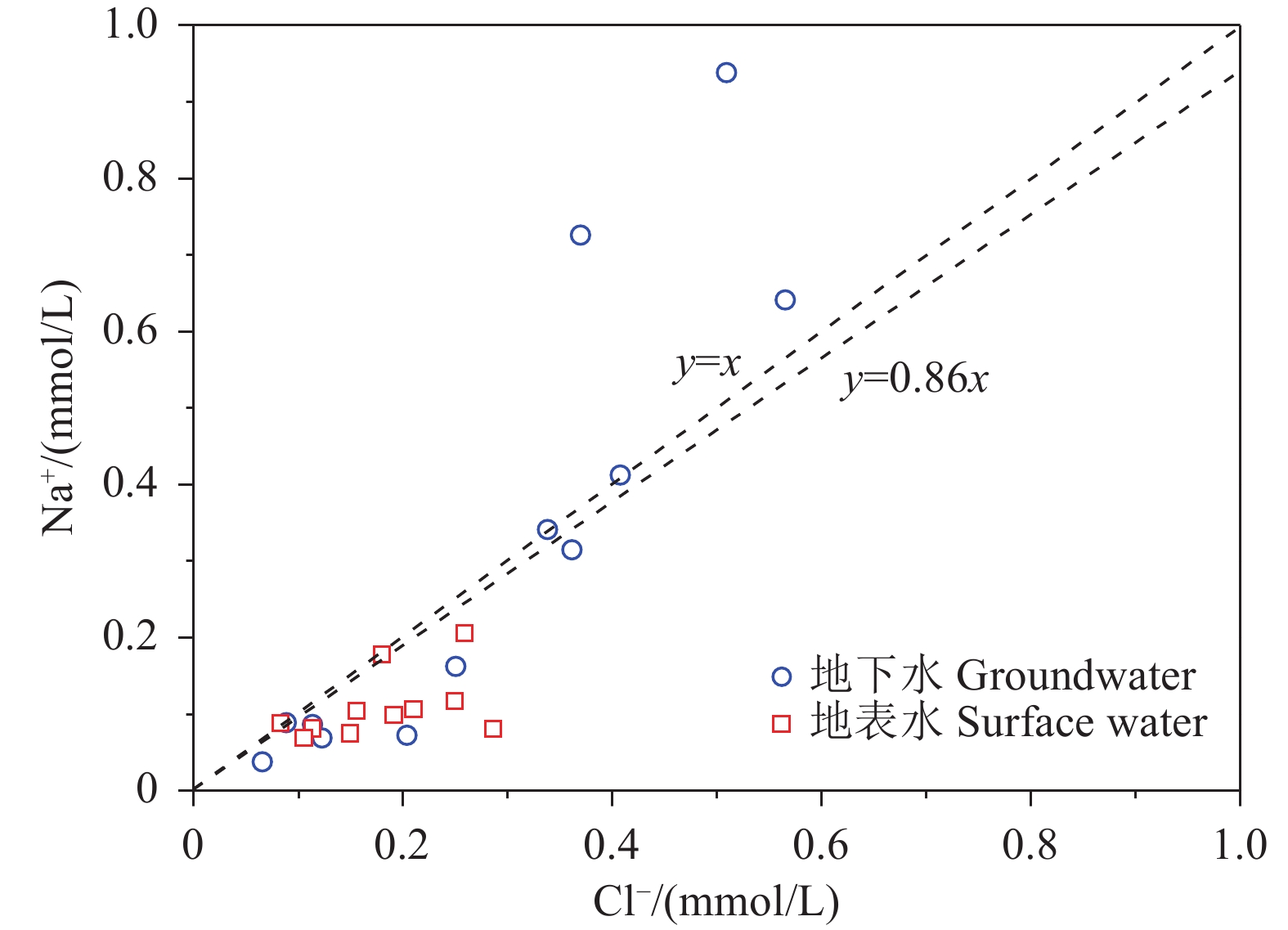
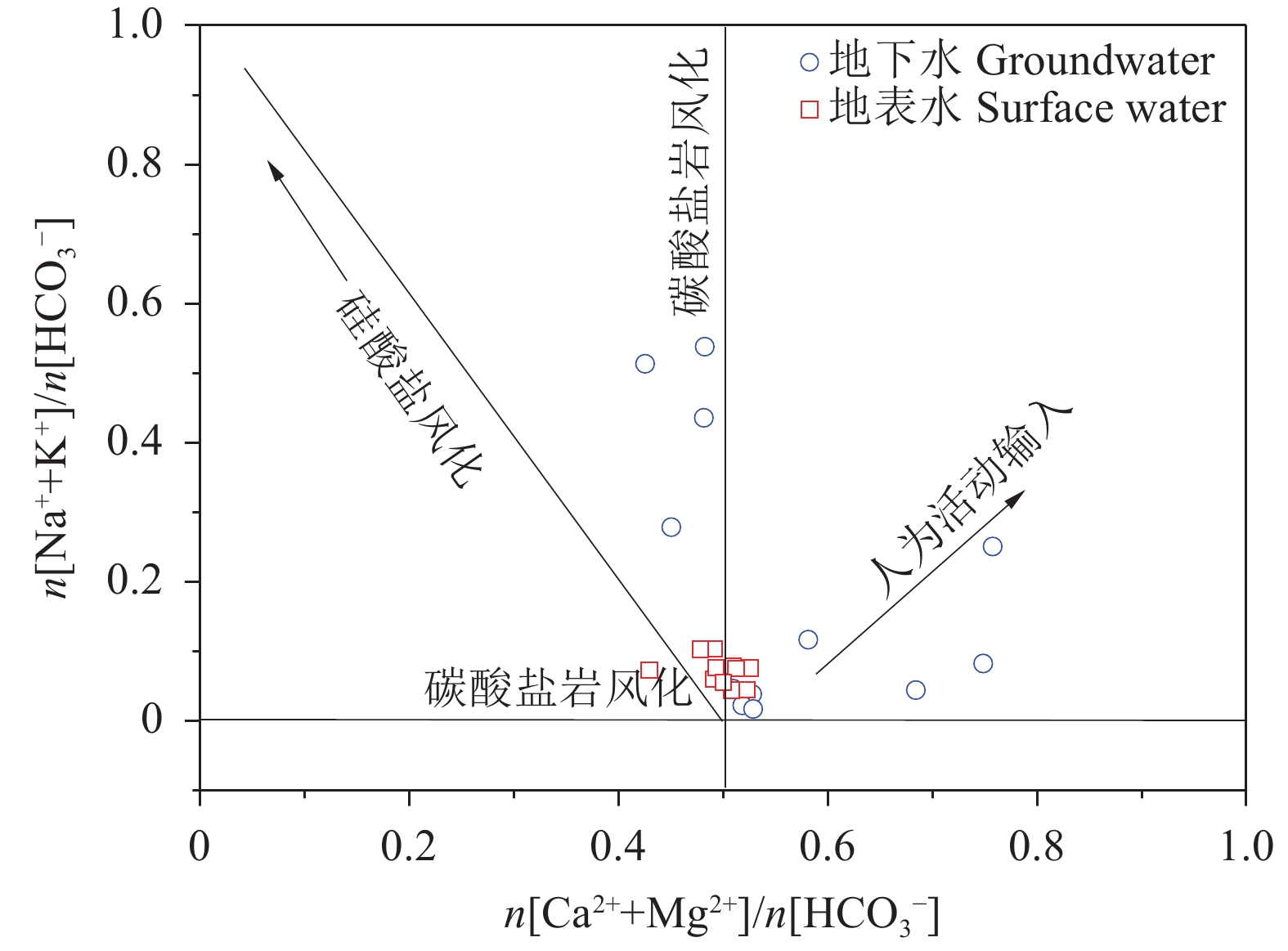
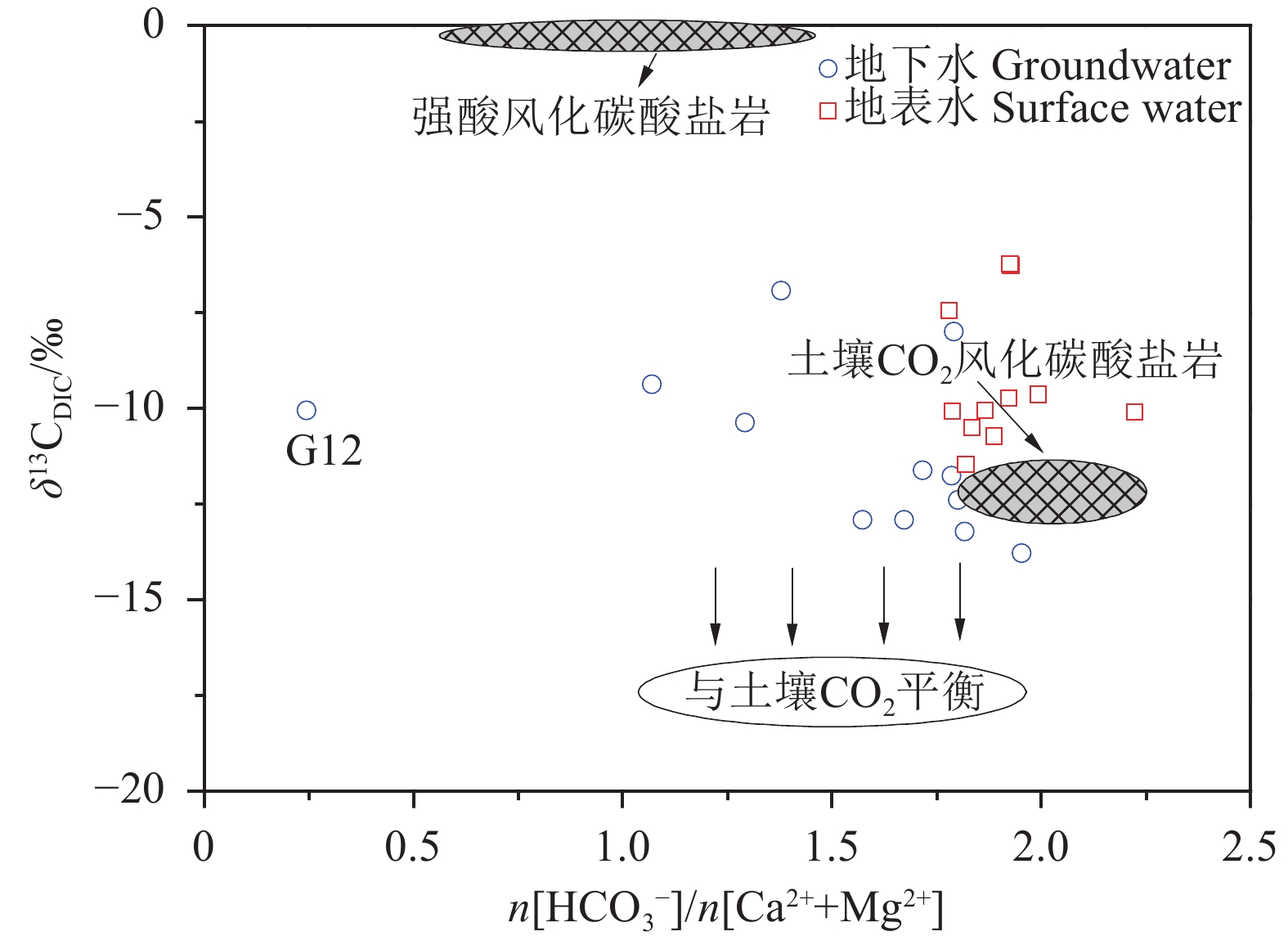
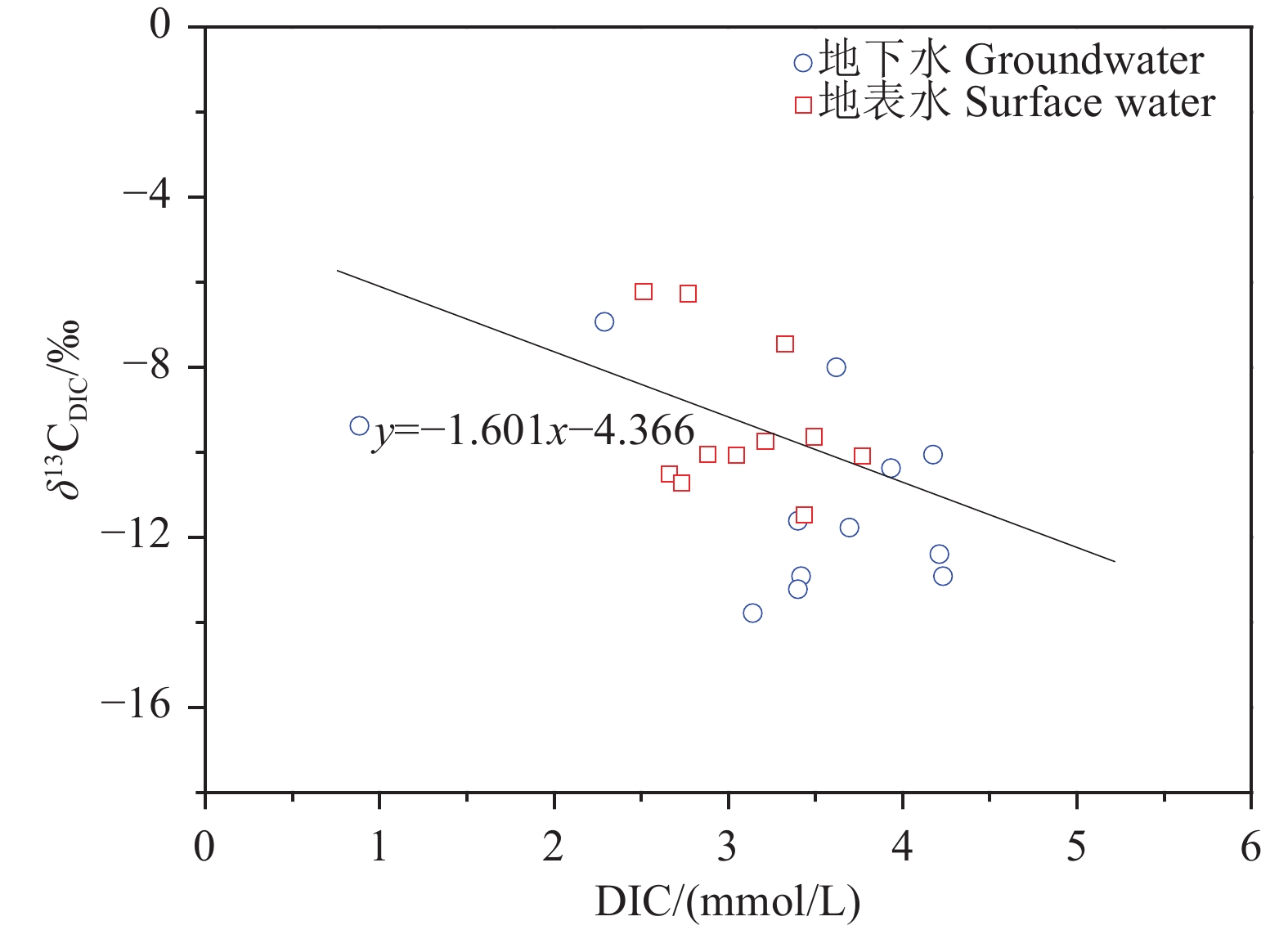
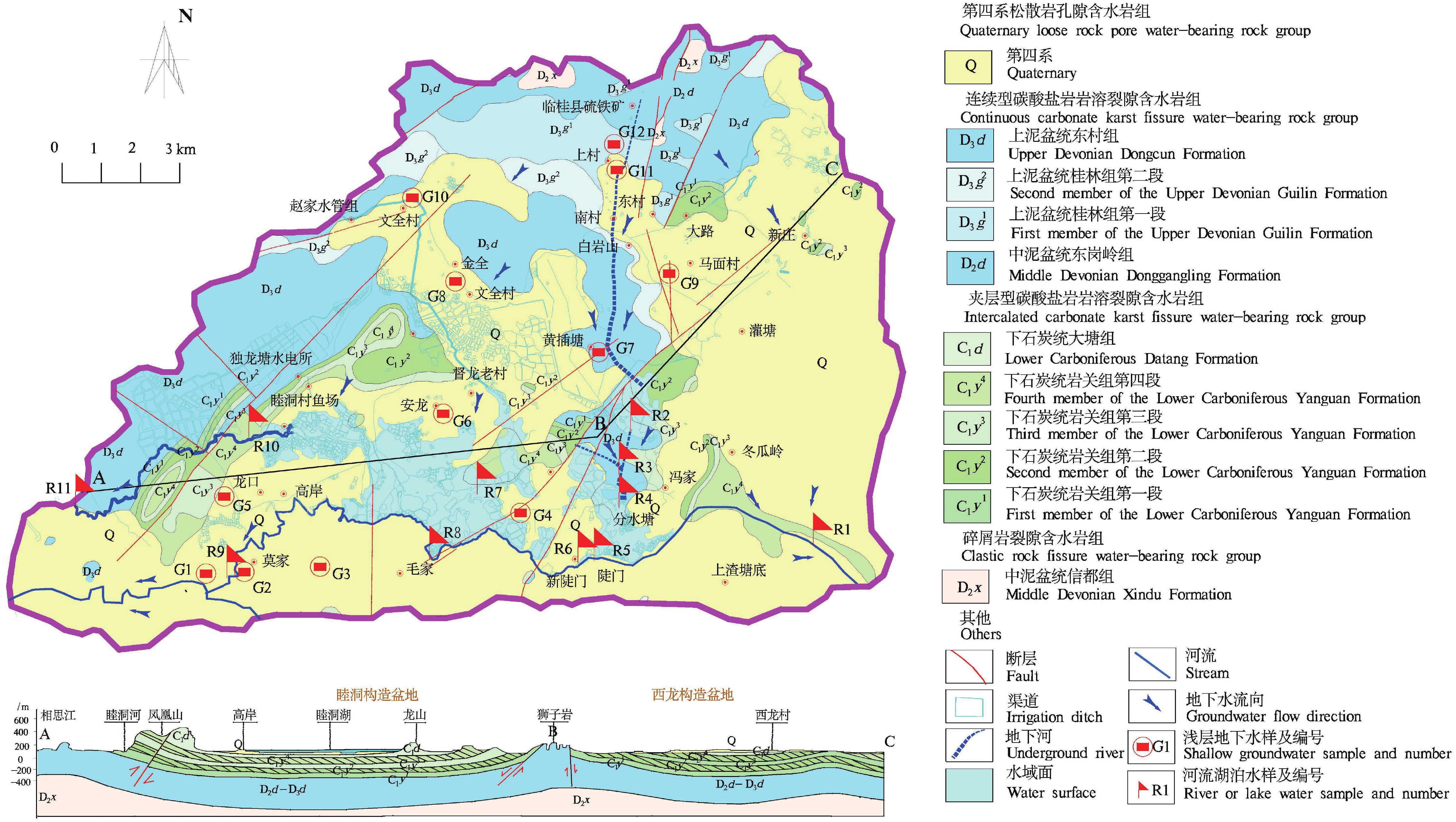
研究结果 会仙岩溶湿地大部分的地下水和地表水水化学类型为Ca−HCO3。湿地水体Ca2+、Mg2+与HCO3−主要来源于碳酸盐岩溶蚀;NO3−主要来源为农业化肥和土壤有机氮的硝化;K+、Na+和Cl−主要来源为化肥、粪肥和污水等;SO42−主要来源为酸雨和硫铁矿的氧化。湿地地下水中DIC主要来源于土壤CO2和碳酸盐岩矿物的溶解,据同位素质量平衡,计算结果显示约46%来自于土壤CO2,约54%来源于矿物本身的贡献。会仙岩溶湿地不完全是CO2参与下碳酸盐岩风化的结果,含硫矿物、酸雨和人类活动来源的H2SO4作为侵蚀介质也参与碳酸盐岩的风化,此外,农业输入还原态氮肥的硝化作用不容忽视。湿地地表水中DIC主要来源于地下水,湿地地表水中δ13CDIC值受水生植物的光合作用和CO2脱气的影响,组成较地下水相对富集偏正。
结论 水化学和δ13CDIC可以帮助理解岩溶湿地的风化和生物地球化学过程,同时还应结合湿地水文地质和人为活动等条件才能提供更准确的信息。
Abstract:This paper is the result of hydrogeological survey engineering.
Objective In order to determine the sources and control factors of main ions and dissolved inorganic carbon (DIC) in water of the Huixian karst wetland,
Methods groundwater and surface water samples were collected from the Huixian karst wetland to analyze the hydrochemical ions and dissolved inorganic carbon isotope(δ13CDIC).
Results The Ca−HCO3 water was identified as a main hydrochemical type in most water samples of the Huixian karst wetland. The dissolution of carbonate rock was primary contributor to Ca2+, Mg2+ and HCO3−, while NO3− was mainly derived from synthetic fertilizers and soil organic nitrogen. Moreover, K+, Na+, and Cl− were driven by the mixed inputs of synthetic fertilizer, manure, and sewage, and the acid rain and pyrite oxidation contributed more to karst water SO42−. Further, karst water DIC was respectively derived from the soil CO2 with the contribution rate of 46% and from the carbonate minerals with the contribution rate of 54% according to the obtained result from isotopic mass balance. In addition to the H2CO3 produced from CO2 and H2O, the H2SO4 derived from sulfur−containing minerals, acid rain, and anthropogenic emissions was involved in carbonate weathering in the Huixian karst wetland. Additionally, microbial nitrification processes of the reduced nitrogen fertilizers could be also ignored in the study area. For the surface water, the DIC was mainly derived from groundwater recharges, and the value of δ13CDIC was affected by the photosynthesis of aquatic plants and CO2 degassing, thereby resulting in the more enrichment of δ13CDIC compared with that in groundwater.
Conclusions The obtained results provided insights into the understanding of minerals weathering and biogeochemical processes, and also highlighted the control factors of hydrogeological conditions and human activities in precisely determining hydrochemical mechanisms in the karst wetland.
-

-
表 1 湿地水体水化学及溶解无机碳同位素参数
Table 1. Hydrochemical and dissolved inorganic carbon isotope parameters of wetland water
水体类型 编号 pH Ca2+/
(mg/L)Mg2+/
(mg/L)Na+/
(mg/L)K+/
(mg/L)Cl−/
(mg/L)SO42−/
(mg/L−)NO3−/
(mg/L)HCO3−/
(mg/L)TDS/
(mg/L)δ13CDIC/
‰lg
(pCO2)SIC 地下水 G1 7.20 76.89 6.02 7.85 2.25 12.00 15.35 37.18 208.50 268.16 −12.92 −1.61 −0.16 G2 7.35 73.45 4.43 1.99 3.11 4.04 16.46 3.11 220.90 224.25 −8.00 −1.58 −0.15 G3 7.49 55.62 5.22 16.69 5.82 13.13 20.60 4.66 191.60 227.73 −13.79 −1.64 −0.33 G4 6.96 22.81 6.21 3.73 2.34 8.89 9.94 31.03 54.10 117.72 −9.38 −2.17 −1.19 G5 7.46 61.74 2.84 1.67 1.12 7.24 6.63 47.94 139.75 202.48 −6.93 −1.77 −0.40 G6 7.44 81.25 11.94 14.75 63.80 20.08 48.49 79.24 258.10 453.77 −12.92 −1.52 −0.09 G7 7.50 78.88 8.80 9.49 55.45 14.47 30.85 65.28 256.97 404.94 −12.40 −1.52 −0.09 G8 7.13 112.70 5.47 7.24 0.35 12.84 6.32 116.75 240.06 386.72 −10.38 −1.55 0.04 G9 7.35 67.26 4.54 21.58 31.44 18.08 47.92 26.47 207.38 329.30 −13.22 −1.61 −0.24 G10 7.49 80.31 1.46 2.04 2.09 3.14 10.74 8.21 225.41 228.88 −11.77 −1.57 −0.10 G11 7.44 70.71 5.10 0.87 0.82 2.33 16.53 1.43 207.38 207.40 −11.62 −1.61 −0.19 G12 7.09 269.25 246.70 1.58 1.00 4.36 1374.8 28.70 254.72 2059.71 −10.06 −1.58 0.11 平均值 7.33 87.57 25.73 7.46 14.13 10.05 133.72 37.5 205.41 425.92 −11.12 −1.64 −0.23 地表水 R1 7.54 59.91 6.11 4.74 6.04 9.20 8.31 <0.05 213.02 213.12 −9.63 −1.59 −0.25 R2 7.55 65.15 5.75 1.72 2.95 5.32 11.92 <0.05 202.87 201.98 −7.45 −1.61 −0.23 R3 7.37 54.20 4.56 1.58 2.31 3.74 7.66 2.18 175.82 170.89 −10.05 −1.67 −0.36 R4 7.52 49.49 4.82 1.87 3.33 4.03 7.59 1.44 169.06 163.31 −6.27 −1.69 −0.41 R5 7.35 50.23 4.62 2.02 2.49 2.96 7.76 2.00 166.80 161.52 −10.73 −1.70 −0.41 R6 7.42 62.56 7.78 4.08 3.72 6.39 12.99 1.44 209.63 211.98 −11.47 −1.60 −0.24 R7 7.32 42.50 5.82 2.39 6.10 5.54 6.78 1.46 153.28 152.64 −6.22 −1.73 −0.51 R8 7.15 52.29 9.56 2.69 4.50 8.87 9.33 <0.05 185.96 189.35 −10.07 −1.65 −0.36 R9 7.57 53.54 8.56 1.86 7.59 10.16 8.50 <0.05 229.92 226.65 −10.09 −1.56 −0.26 R10 7.26 46.19 7.09 2.27 3.99 6.80 8.13 <0.05 162.30 164.10 −10.51 −1.71 −0.46 R11 7.38 53.50 8.05 2.44 5.48 7.47 8.42 <0.05 196.11 195.30 −9.74 −1.63 −0.32 平均值 7.40 53.60 6.61 2.51 4.41 6.41 8.85 − 187.71 186.44 −9.29 −1.65 −0.35 表 2 地下水中DIC不同来源所占比例
Table 2. Proportion of different sources of DIC in groundwater
水体类型 编号 δ13CDIC(V−PDB,‰) lg(pCO2) SIc 土壤CO2源 碳酸盐岩源 地下水 G1 −12.92 −1.61 −0.16 0.53 0.47 G2 −8.00 −1.58 −0.15 0.33 0.67 G3 −13.79 −1.64 −0.33 0.57 0.43 G4 −9.38 −2.17 −1.19 0.38 0.62 G5 −6.93 −1.77 −0.40 0.28 0.72 G6 −12.92 −1.52 −0.09 0.53 0.47 G7 −12.40 −1.52 −0.09 0.51 0.49 G8 −10.38 −1.55 0.04 0.43 0.57 G9 −13.22 −1.61 −0.24 0.54 0.46 G10 −11.77 −1.57 −0.10 0.48 0.52 G11 −11.62 −1.61 −0.19 0.48 0.52 G12 −10.06 −1.58 0.11 0.41 0.59 平均值 −11.12 −1.64 −0.23 0.46 0.54 -
[1] Abusaada M, Sauter M. 2013. Studying the flow dynamics of a karst aquifer system with an equivalent porous medium model[J]. Groundwater, 51(4): 641−650. doi: 10.1111/j.1745-6584.2012.01003.x
[2] Bade D L, Carpenter S R, Cole J J, Hanson P C, Hesslein R H. 2004. Controls of δ13C−DIC in lakes: Geochemistry, lake metabolism, and morphometry[J]. Limnology and Oceanography, 49(4): 1160−1172. doi: 10.4319/lo.2004.49.4.1160
[3] Cao Jianhua, Zhou Li, Yang Hui, Lu Qian, Kang Zhiqiang. 2011. Comparison of carbon transfer between forest soils in karst and clasolite areas and the karst carbon sink effect in Maocun village of Guilin[J]. Quaternary Sciences, 31(3): 431−437 (in Chinese with English abstract).
[4] Cao Xingxing. 2016. Study on Geochemical Process of Karst Wetland Basin Based on Changes of Water Chemistry and Stable Isotope[D]. Guiyang: Guizhou University, 1−116 (in Chinese with English abstract).
[5] Chen Ling, Wang Zhongliang. 2012. Applications of carbon isotopic method in wetland carbon cycle and related research advances[J]. Chinese Journal of Ecology, 31(7): 1862−1869 (in Chinese with English abstract).
[6] Cosford J, Qing H R, Mattey D, Eglington B, Zhang M L. 2009. Climatic and local effects on stalagmite δ13C values at Lianhua Cave, China[J]. Palaeogeography, Palaeogeography, Palaeogeography, 280(1): 235−244.
[7] Han G L, Liu C Q. 2004. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst−dominated terrain, Guizhou Province, China[J]. Chemical Geology, 204(1/2): 1−21.
[8] Han Guilin, Liu Congqiang. 2005. Hydrogeochemistry of rivers in Guizhou Province, China: Constraints on crustal weathering in karst terrain[J]. Advances in Earth Science, 20(4): 394−406 (in Chinese with English abstract).
[9] Huang Qibo, Qin Xiaoqun, Tang Pingping, Liu Pengyu. 2013. The characteristic and significance of carbon isotope (δ13CDIC) and oxygen isotope (δ18O) value in different type of karst water in Guilin[J]. Geochimica, 42(1): 64−72 (in Chinese with English abstract).
[10] Jiang Y J, Zhang C, Yuan D X, Zhang G, He R S. 2008. Impact of land use change on groundwater quality in a typical karst watershed of southwest China: A case study of the Xiaojiang watershed, Yunnan Province[J]. Hydrogeology Journal, 16(4): 727−735. doi: 10.1007/s10040-007-0259-9
[11] Jiang Y J. 2013. The contribution of human activities to dissolved inorganic carbon fluxes in a karst underground river system: evidence from major elements and δ13CDIC in Nandong, Southwest China[J]. Journal of Contaminant Hydrology, 152: 1−11. doi: 10.1016/j.jconhyd.2013.05.010
[12] Jin Yang, Jiang Yuehua, Dong Xianzhe, Yang Guoqiang, Liu Hongying, Lei Changzheng, Zhou Quanping, Zhang Hong, Mei Shijia, Yang Hui, Lü Jinsong, Li Yun. 2022. Chemical characteristics and eco−environmental effect of groundwater in Ningbo Plain, Zhejiang Province[J]. Geology in China, 49(5): 1527−1542 (in Chinese with English abstract).
[13] Li Jun, Liu Congqiang, Li Longbo, Li Siliang, Wang Baoli, Chetelat B. 2010. The impacts of chemical weathering of carbonate rock by sulfuric acid on the cycling of dissolved inorganic carbon in Changjiang River water[J]. Geochimica, 39(4): 305−313 (in Chinese with English abstract).
[14] Li Q Y, Wu J L, Shen B B, Zeng H A, Li Y H. 2018. Water chemistry and stable isotopes of different water types in Tajikistan[J]. Environmental Processes, 5(S1): 127−137. doi: 10.1007/s40710-018-0312-9
[15] Li S L, Liu C Q, Lang Y C, Tao F, Zhao Z Q, Zhou Z H. 2008. Stable carbon isotope biogeochemistry and anthropogenic impacts on karst ground water, Zunyi, Southwest China[J]. Aquatic Geochemistry, 14(3): 211−221. doi: 10.1007/s10498-008-9033-4
[16] Li S L, Liu C Q, Li Jun, Lang Y C, Ding H, Li L B. 2010. Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints[J]. Chemical Geology, 277(3/4): 301−309. doi: 10.1016/j.chemgeo.2010.08.013
[17] Li X D, Liu C Q, Harue M, Li S L, Liu X L. 2010. The use of environmental isotopic (C, Sr, S) and hydrochemical tracers to characterize anthropogenic effects on karst groundwater quality: A case study of the Shuicheng Basin, SW China[J]. Applied Geochemistry, 25(12): 1924−1936. doi: 10.1016/j.apgeochem.2010.10.008
[18] Li Zhuang, Su Jingwen, Dong Changchun, Ye Yonghong, Yang Yang. 2022. Hydrochemistry characteristics and evolution mechanisms of the groundwater in Dangtu area, Ma'anshan City, Anhui Province[J]. Geology in China, 49(5): 1509−1526 (in Chinese with English abstract).
[19] Liu Congqiang, Jiang Yingkui, Tao Faxing, Lang Yunchao, Li Siliang. 2008. Chemical weathering of carbonate rocks by sulfuric acid and the carbon cycling in Southwest China[J]. Geochimica, 37(4): 404−414 (in Chinese with English abstract).
[20] Meybeck M. 1982. Carbon, nitrogen, and phosphorus transport by world rivers[J]. American Journal of Science, 282(4): 401−450. doi: 10.2475/ajs.282.4.401
[21] Pant R R, Zhang F, Rehman F U, Wang G X, Ye M, Zeng C, Tang H D. 2018. Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal[J]. Science of the Total Environment, 622(1): 770−782.
[22] Peng Jiantang, Hu Ruizhong. 2001. Carbon and oxygen isotope systematics in the Xikuangshan giant antimony deposit, central Hunan[J]. Geological Review, 47(1): 34−41 (in Chinese with English abstract).
[23] Qiu Xiaojuan, Yan Zhiwei, Wei Lanlan, Qin Zhuoping, Huang Xiangping. 2012. Study on chemical characteristics of atmospheric precipitation in GUT[J]. Environmental Science and Management, 37(11): 68−71 (in Chinese with English abstract).
[24] Ren Kun, Pan Xiaodong, Zeng Jie, Jiao Youjun, Peng Cong, Liang Jiapeng. 2019. Geochemical characteristics and ecological significance of carbon isotopes in groundwater under the influence of different land use types in Karst Areas[J]. Environmental Science, 40(10): 4523−4531 (in Chinese with English abstract).
[25] Semhi K, Suchet P A, Clauer N, Probst J. 2000. Impact of nitrogen fertilizers on the natural weathering−erosion processes and fluvial transport in the Garonne basin[J]. Applied Geochemistry, 15(6): 865−878. doi: 10.1016/S0883-2927(99)00076-1
[26] Song Tao, Zou Shengzhang, Zhang Liankai, Zhou Changsong, Zhao Yi, Shen Lina. 2020. Preliminary study on the construction of evaluation index system of karst wetland degradation[J]. Carsologica Sinica, 39(5): 673−681(in Chinese with English abstract).
[27] Stern J, Wang Y, Gu B, Newman J. 2007. Distribution and turnover of carbon in natural and constructed wetlands in the Florida Everglades[J]. Applied Geochemistry, 22(9): 1936−1948. doi: 10.1016/j.apgeochem.2007.04.007
[28] Sun H, Han J, Li D, Zhang S R, Lu X X. 2010. Chemical weathering inferred from riverine water chemistry in the lower Xijiang Basin, South China[J]. Science of the Total Environment, 408(20): 4749−4760. doi: 10.1016/j.scitotenv.2010.06.007
[29] Tang Wenkui, Tao Zhen, Gao Quanzhou, Mao Hairuo, Jiang Guanghui, Jiao Shulin, Zheng Xiongbo, Zhang Qianzhu, Ma Zanwen. 2014. Biogeochemical processes of the major ions and dissolved inorganic carbon in the Guijiang River[J]. Environmental Science, 35(6): 2099−2107 (in Chinese with English abstract).
[30] Tang Xiwen, Wu Jinkui, Xue Liyang, Zhang Mingquan, Frauke Barthold, Lutz Breuer, Hans−Georg Frede. 2014. Major ion chemistry of surface water in the Xilin River Basin and the possible controls[J]. Environmental Science, 35(1): 131−142 (in Chinese with English abstract).
[31] Wang B, Lee X Q, Yuan H L, Zhou H, Cheng H G, Cheng J Z, Zhou Z H, Xing Y, Fang B, Zhang L K, Yang F. 2012. Distinct patterns of chemical weathering in the drainage basins of the Huanghe and Xijiang River, China: Evidence from chemical and Sr−isotopic composition[J]. Journal of Asian Earth Sciences, 59(S1): 219−230.
[32] Wu P, Tang C, Zhu L, Liu C Q, Cha X F, Tao X Z. 2009. Hydrogeochemical characteristics of surface water and groundwater in the karst basin, southwest China[J]. Hydrological Processes, 23(14): 2012−2022. doi: 10.1002/hyp.7332
[33] Yan Hui, Li Zhongxuan, Chen Jie. 2011. Seasonal variations in dissolved inorganic carbon and δ13C of the Huaxi River[J]. Earth and Environment, 39(3): 300−304 (in Chinese with English abstract).
[34] Zeng Haiao, Wu Jinglu, Liu Wen, Ma Long, Jilili Abuduwaili, Saparov A S. 2013. Characteristics of hydrochemistry and hydrogen, oxygen isotopes of waters in Kazakhstan[J]. Arid Land Geography, 36(4): 662−668 (in Chinese with English abstract).
[35] Zhang Cheng. 2015. Diel aqueous chemistry and biogeochemical processes in streams of karst areas[J]. Carsologica Sinica, 34(1): 1−8 (in Chinese with English abstract).
[36] Zhao Yi, Zou Shengzhang, Shen Haoyong, Zhou Changsong, Fan Lianjie, Zhu Danni, Li Jun. 2021. Dynamic characteristics and equilibrium of water level of the karst groundwater system beneath the Huixian wetland[J]. Carsologica Sinica, 40(2): 325−333 (in Chinese with English abstract).
[37] Zhou Jinmei, Jiang Zhongcheng, Xu Guangli, Qin Xiaoqun, Huang Qibo, Zhang Liankai. 2019. Major ionic characteristics and controlling factors of karst groundwater at Xiangshui, Chongzuo[J]. Environmental Science, 40(5): 2143−2151 (in Chinese with English abstract).
[38] Zhu Z J, Chen J A, Zeng Y, Li H, Yan H, Ren S C. 2011. Research on the carbon isotopic composition of organic matter from Lake Chenghai and Caohai Lake sediments[J]. Chinese Journal of Geochemistry, 30(1): 107−113.
[39] 曹建华, 周莉, 杨慧, 卢茜, 康志强. 2011. 桂林毛村岩溶区与碎屑岩区林下土壤碳迁移对比及岩溶碳汇效应研究[J]. 第四纪研究, 31(3): 431−437. doi: 10.3969/j.issn.1001-7410.2011.03.05
[40] 曹星星. 2016. 基于水化学与稳定同位素的岩溶湿地流域地球化学过程研究[D]. 贵阳: 贵州大学,1−116.
[41] 陈玲, 王中良. 2012. 碳同位素在湿地碳循环研究中的应用及进展[J]. 生态学杂志, 31(7): 1862−1869.
[42] 韩贵琳, 刘丛强. 2005. 贵州喀斯特地区河流的研究—碳酸盐岩溶解控制的水文地球化学特征[J]. 地球科学进展, 20(4): 394−406.
[43] 黄奇波, 覃小群, 唐萍萍, 刘朋雨. 2013. 桂林地区不同类型岩溶地下水中δ13CDIC、δ18O的特征及意义[J]. 地球化学, 42(1): 64−72. doi: 10.3969/j.issn.0379-1726.2013.01.008
[44] 金阳, 姜月华, 董贤哲, 杨国强, 刘红樱, 雷长征, 周权平, 张鸿, 梅世嘉, 杨辉, 吕劲松, 李云. 2022. 浙江宁波平原地下水水化学特征及其生态环境效应[J]. 中国地质, 49(5): 1527−1542.
[45] 李军, 刘丛强, 李龙波, 李思亮, 王宝利, Chetelat B. 2010. 硫酸侵蚀碳酸盐岩对长江河水DIC循环的影响[J]. 地球化学, 39(4): 305−313.
[46] 李状, 苏晶文, 董长春, 叶永红, 杨洋. 2022. 安徽马鞍山市当涂地区地下水水化学特征及演化机制[J]. 中国地质, 49(5): 1509−1526.
[47] 刘丛强, 蒋颖魁, 陶发祥, 郎赟超, 李思亮. 2008. 西南喀斯特流域碳酸盐岩的硫酸侵蚀与碳循环[J]. 地球化学, 37(4): 404−414. doi: 10.3321/j.issn:0379-1726.2008.04.014
[48] 彭建堂, 胡瑞忠. 2001. 湘中锡矿山超大型锑矿床的碳、氧同位素体系[J]. 地质评论, 47(1): 34−41.
[49] 邱晓娟, 闫志为, 韦兰兰, 覃卓萍, 黄香萍. 2012. 桂林大气降水化学特征及其经树木枝叶淋滤后的变化[J]. 环境科学与管理, 37(11): 68−71. doi: 10.3969/j.issn.1673-1212.2012.11.015
[50] 任坤, 潘晓东, 曾洁, 焦友军, 彭聪, 梁嘉鹏. 2019. 岩溶区不同土地利用下地下水碳同位素地球化学特征及生态意义[J]. 环境科学, 40(10): 4523−4531.
[51] 宋涛, 于晓英, 邹胜章, 张连凯, 刘朋雨, 赵一, 沈利娜. 2020. 岩溶湿地退化评价指标体系构建初探[J]. 中国岩溶, 39(5): 673−681.
[52] 唐文魁, 陶贞, 高全洲, 毛海若, 姜光辉, 焦树林, 郑雄波, 张乾柱, 马赞文. 2014. 桂江主要离子及溶解无机碳的生物地球化学过程[J]. 环境科学, 35(6): 2099−2107.
[53] 唐玺雯, 吴锦奎, 薛丽洋, 张明泉, Frauke B, Lutz B, Hans G F. 2014. 锡林河流域地表水水化学主离子特征及控制因素[J]. 环境科学, 35(1): 131−142.
[54] 闫慧, 李中轩, 陈杰. 2011. 花溪河水溶解无机碳同位素的季节变化[J]. 地球与环境, 39(3): 300−304.
[55] 曾海鳌, 吴敬禄, 刘文, 马龙, 吉力力·阿不都外力, Saparov A S. 2013. 哈萨克斯坦东部水体氢、氧同位素和水化学特征[J]. 干旱区地理, 36(4): 662−668.
[56] 章程. 2015. 岩溶区河流水化学昼夜变化与生物地球化学过程[J]. 中国岩溶, 34(1): 1−8. doi: 10.11932/karst20150101
[57] 赵一, 邹胜章, 申豪勇, 周长松, 樊连杰, 朱丹尼, 李军. 2021. 会仙湿地岩溶地下水系统水位动态特征与均衡分析[J]. 中国岩溶, 40(2): 325−333.
[58] 周巾枚, 蒋忠诚, 徐光黎, 覃小群, 黄奇波, 张连凯. 2019. 崇左响水地区岩溶地下水主要离子特征及控制因素[J]. 环境科学, 40(5): 2143−2151.
-



 下载:
下载: