Migration behavior of organic pollutants in typical sediments of the Yangtze River delta urban agglomeration
-
摘要:
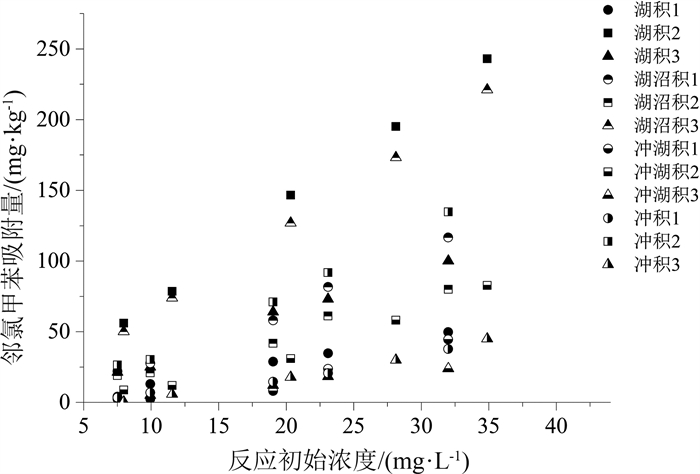
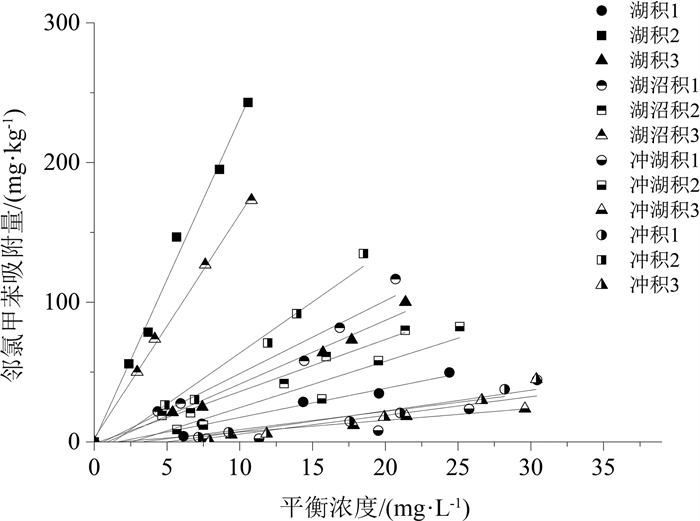
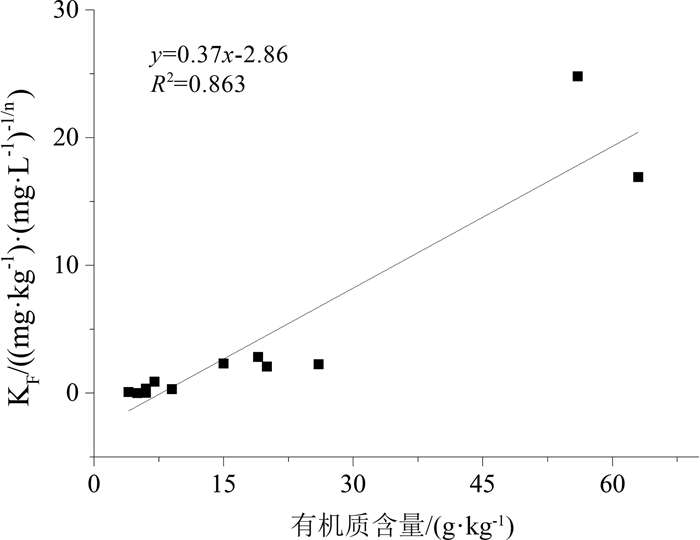
长江三角洲城市群地区是中国沉积类型种类相对多、岩性类型复杂的地质区域。改革开放以来该地区化工企业快速发展, 使有机化工产品泄漏到土壤和沉积物中的风险加大, 对环境构成了较大的安全隐患, 因此, 掌握污染物在土壤和沉积物中的迁移转化规律对后续开发利用具有实用意义。采集并分析了长江三角洲城市群4种代表性的沉积物, 以邻氯甲苯为代表开展了有机污染物在沉积物中迁移行为的研究, 并利用SPSS软件建立邻氯甲苯吸附量与沉积物理化性质之间的相关方程。研究结果表明: 长江三角洲城市群湖积相、湖沼积相、冲湖积相和冲积相沉积物的有机质含量直接控制其对邻氯甲苯的吸附阻滞能力, 土壤的pH、矿物成分、阳离子交换量对邻氯甲苯的吸附能力影响不大, 苏州的湖积和湖沼积沉积物中有机质含量较高(7~63 g/kg), 其对邻氯甲苯的吸附阻滞能力较强, 而南京、镇江等地的冲积沉积物和常州、江阴南部的冲湖积沉积物中有机质含量较低(4~15 g/kg), 对邻氯甲苯的吸附能力较弱, 使污染物更易迁移; Freundlich模型能够较好地拟合不同种类沉积物对邻氯甲苯的等温吸附过程, 表征吸附强度的系数KF与沉积物的有机质含量呈显著正相关, 有机质含量越大, KF值越大, 沉积物对邻氯甲苯的吸附强度越强, 邻氯甲苯的等温吸附过程线性程度越高。研究成果将有利于预测有机污染物在长江三角洲城市群地区不同种类沉积物中的吸附行为, 为治理污染和调整工业布局提供理论依据。
Abstract:The Yangtze River delta urban agglomeration is a geological region with many sedimentary types and complex lithology types.Since the reform and opening up, the rapid development of chemical enterprises in this area has increased the risk of leakage of organic chemical products into soil and sediment.It poses a great potential safety hazard to the environment.Therefore, mastering the migration and transformation of pollutants in soil and sediment has practical significance for the subsequent development and utilization of these plots.The organic chemical industry is well developed in the Yangtze River delta urban agglomeration.Organic pollution of soil and groundwater in brownfield is serious.The physical and chemical properties of the soil in brownfield control the extent of contamination.Four representative sediments from the Yangtze River delta urban agglomeration were collected and analyzed in this paper.The physical and chemical properties of sediment affecting o-chlorotoluene migration were analyzed based on the study of differentiation between sediments and the statistic software SPSS.The result of research shows that the Yangtze River delta urban agglomeration can be divided into alluvial, lacustrine, lacustrine-swamp and alluvial sedimentary areas.The adsorption capacity of o-chlorotoluene was directly controlled by the organic content of the sediment matrix.The cation exchange capacity, mineral composition and pH of soil had little effect on the adsorption capacity of o-chlorotoluene.The content of organic matter in the lacustrine and lacustrine-swamp sediments in Suzhou is relatively high(7~63 g/kg), and its adsorption capacity of o-chlorotoluene is strong.In Nanjing, Zhenjiang and south of Changzhou, Jiangyin where the alluvial sediments and alluvial-lacustrine sediments developed, the adsorption strength of o-chlorotoluene to the sediments is weak as its low organic matter content(4~15 g/kg), making the pollutants easier to migrate.The Freundlich model can fit the isothermal adsorption process of o-chlorotoluene on the four kinds of sediment samples well.The parameters KF characterizing the adsorption strength in the model was significantly positive correlated with the content of organic matter in sediments.The higher the organic matter content, the higher the KF value, the stronger the adsorption strength of the sediments to o-chlorotoluene, the higher the linearity of the isothermal adsorption process.The study will be helpful to predict the adsorption behavior of organic pollutants in different sediments in the Yangtze River delta urban agglomeration and provide scientific theoretical basis for controlling pollution and adjusting industrial layout.
-

-
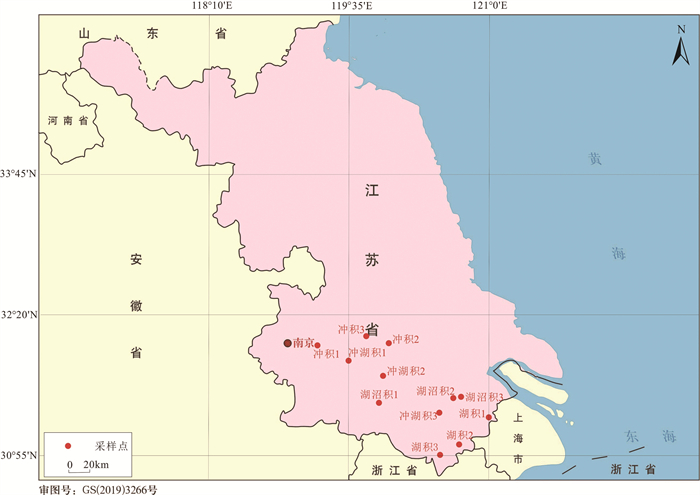
表 1 采样点基本信息
Table 1. Basic information about the sampling points
样品名称 经度/° 纬度/° 高程/m 沉积类型 样品颜色 取样深度/m 湖积1 121.096145 31.326163 2.672 湖积相 黄褐色 2~2.5 湖积2 120.655125 31.031528 3.569 湖积相 灰色 1.5~2.5 湖积3 120.447930 30.925162 4.453 湖积相 灰褐色 2.5 湖沼积1 119.766778 31.455253 3.704 湖沼积相 灰褐色 2 湖沼积2 120.648452 31.493705 2.072 湖沼积相 灰色 2.3 湖沼积3 120.725356 31.496451 4.807 湖沼积相 灰色 2 冲湖积1 119.580182 31.858560 4.775 冲湖积相 黄褐色 2 冲湖积2 119.852093 31.696512 2.357 冲湖积相 黄色 2.5 冲湖积3 120.417739 31.331656 7.198 冲湖积相 黄褐色 2.3 冲积1 119.043096 31.985214 9.132 冲积相 黄色 2.5 冲积2 119.908956 32.020920 4.279 冲积相 黄褐色 2.5~3 冲积3 119.761456 32.130472 3.694 冲积相 黄色 2.3 表 2 实验主要仪器
Table 2. The instruments used in the experiment
仪器名称 型号 生产厂商 叠加式恒温振荡器 HNY-202B 天津欧诺仪器公司 高速离心机 L550 湖南湘仪离心机公司 气相色谱仪 Agilent GC 6820 美国安捷伦公司 电子天平 FA2004B 上海精科天美公司 数显恒温水浴锅 HH-2型号 常州智博瑞仪器制造公司 表 3 沉积物主要理化性质
Table 3. The main physical and chemical properties of sediments
样品名称 pH 有机质/(g·kg-1) CEC含量/(cmol·kg-1) 湖积1 6.87 7 17.31 湖积2 5.99 56 15.59 湖积3 6.73 20 8.39 湖沼积1 6.39 26 14.34 湖沼积2 6.38 19 19.18 湖沼积3 5.66 63 16.61 冲湖积1 6.00 5 17.11 冲湖积2 6.03 9 17.40 冲湖积3 6.99 6 19.33 冲积1 7.09 4 12.02 冲积2 7.42 15 11.51 冲积3 7.29 6 13.11 表 4 沉积物主要矿物成分
Table 4. The main mineral composition of the sediments
% 样品名称 石英 斜长石 微斜长石 赤铁矿 闪石 白云石 方解石 粘土矿物总量 湖积1 47 14 5 3 - - - 30 湖积2 60 13 5 - - - - 22 湖积3 59 17 6 1 - - - 17 湖沼积1 51 13 10 - - - - 26 湖沼积2 54 17 4 - - - - 25 湖沼积3 47 17 4 - 5 - - 28 冲湖积1 52 14 8 - - - - 26 冲湖积2 54 13 4 1 - - - 29 冲湖积3 57 12 6 - - - - 25 冲积1 64 10 5 - - - - 22 冲积2 43 11 5 - 4 4 6 28 冲积3 68 7 4 - - - - 21 表 5 邻氯甲苯动力学模型拟合参数
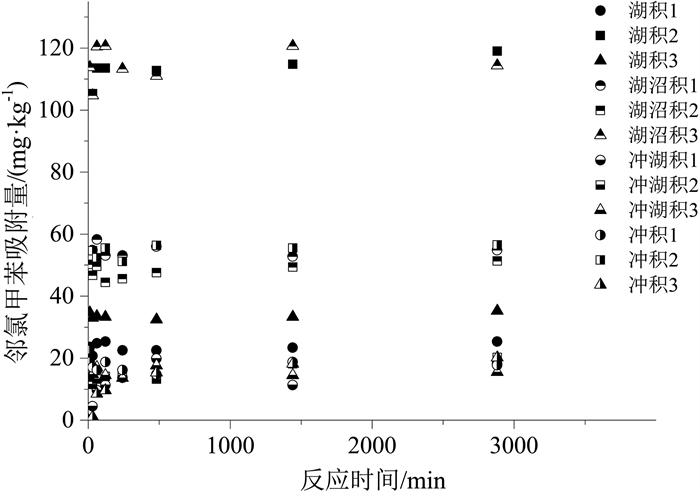
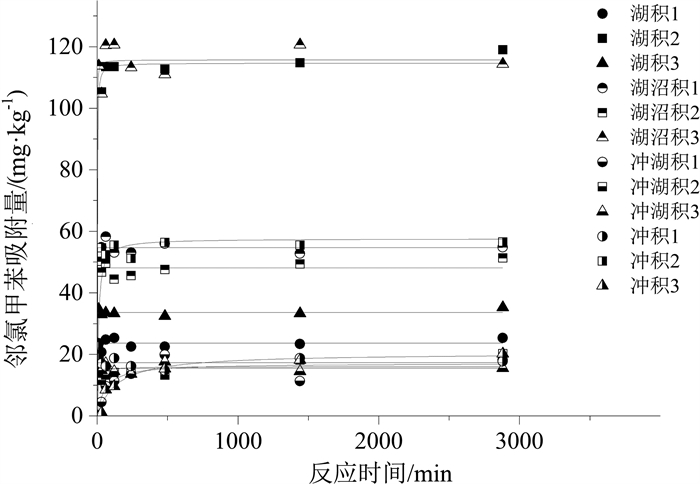
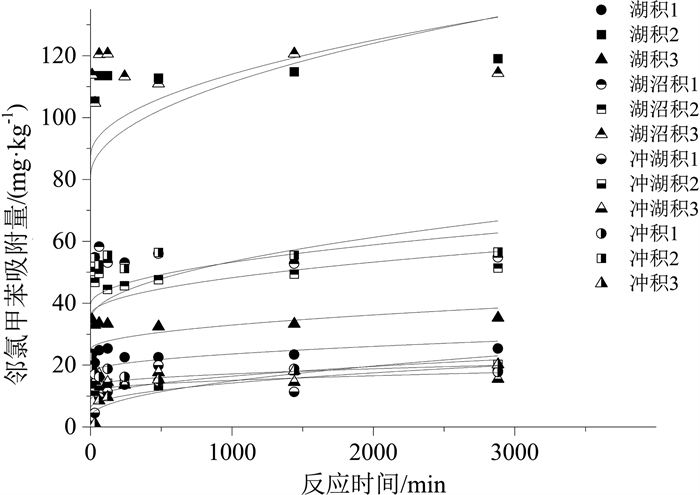
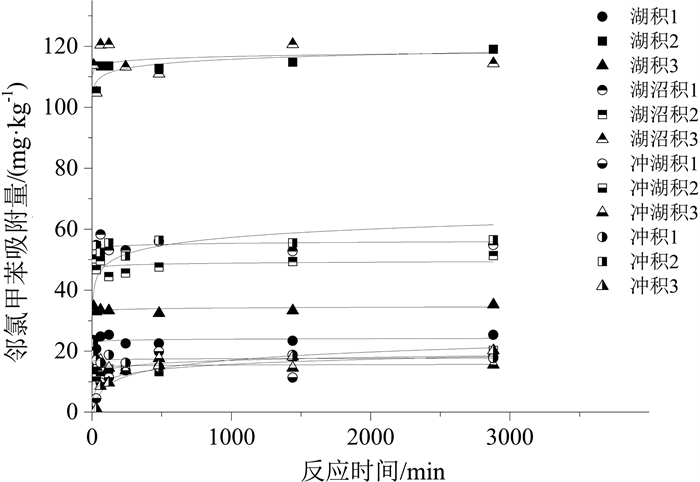
Table 5. Kinetic model fitting parameters of o-chlorotoluene
样品名称 颗粒内扩散模型 准二级动力学模型 Elovich模型 k/(mg·(min1/2· kg)-1) C /(mg·kg-1) R2 k/(kg·(mg· min)-1) qm /(mg·kg-1) R2 a/(mg·(kg· min)-1) b/(kg·mg-1) R2 湖积1 0.19 17.69 0.057 4.6×104 23.71 0.959 2.1 ×1041 4.32 0.962 湖积2 0.96 81.14 0.078 1.4×106 114.72 0.994 1.2×1021 0.47 0.996 湖积3 0.25 25.06 0.019 7.2×1023 33.67 0.993 5.1 ×1044 3.25 0.991 湖沼积1 0.43 39.65 0.023 5.7×1020 54.73 0.989 4.3×1043 1.95 0.985 湖沼积2 0.38 36.12 0.061 4.0×1022 48.15 0.977 5.5×1044 2.26 0.978 湖沼积3 0.84 87.61 0.027 4.4×106 115.75 0.980 1.3×1046 0.97 0.980 冲湖积1 0.25 6.29 0.396 91.1 17.11 0.801 2.01 0.43 0.719 冲湖积2 0.24 8.84 0.520 713.1 16.04 0.814 310.5 0.72 0.938 冲湖积3 0.11 11.52 0.027 3079.5 15.54 0.932 6.4×1032 5.43 0.924 冲积1 0.13 13.19 0.022 6.5×1026 17.31 0.937 9.1×1043 6.25 0.932 冲积2 0.60 34.63 0.182 2.2×104 57.63 0.930 9.1×103 0.26 0.816 冲积3 0.37 3.17 0.796 63.4 20.45 0.959 0.34 0.26 0.956 表 6 邻氯甲苯在不同土样中吸附模型拟合结果
Table 6. The adsorption model fitting results of o-chlorotoluene in different soil samples
土样名称 线性 Freundlich Kd/(L· kg-1) R2 KF/((mg·kg-1) · (mg·L-1) -1/ n) 1/ n R2 湖积1 2.08 0.962 0.89 1.26 0.971 湖积2 23.06 0.991 24.81 0.97 0.991 湖积3 4.55 0.981 2.08 1.25 0.992 湖沼积1 5.21 0.952 2.26 1.28 0.966 湖沼积2 3.73 0.983 2.84 1.09 0.985 湖沼积3 16.03 0.997 16.92 1.00 0.999 冲湖积1 1.34 0.727 0.01 2.72 0.995 冲湖积2 3.32 0.925 0.32 1.72 0.985 冲湖积3 0.84 0.967 0.35 1.25 0.980 冲积1 1.30 0.910 0.08 1.82 0.991 冲积2 7.3 0.967 2.33 1.39 0.995 冲积3 1.48 0.876 0.02 2.30 0.991 表 7 KF、|1-1/n|与沉积物理化性质相关性
Table 7. The relativity between KF, |1-1/n| and physical and chemical properties of sediments
参数 类别 有机质含量 粘土矿物总量 pH CEC KF Pearson相关性 0.929** -0.064 -0.559 0.089 显著性(双侧) 1.3×10-5 0.843 0.059 0.783 N 12 12 12 12 |1-1/n| Pearson相关性 -0.589* -0.084 0.136 -0.083 显著性(双侧) 0.044 0.794 0.673 0.798 N 12 12 12 12 注:**表示在0.01水平(双侧)上显著相关; *表示在0.05水平(双侧)上显著相关 -
[1] Wu K, Zheng K, Xiong L B, et al. Efficient synthesis of an antiviral drug intermediate using an enhanced short-chain dehydrogenase in an aqueous-organic solvent system[J]. Applied Microbiology and Biotechnology, 2019, 103(11): 4417-4427. doi: 10.1007/s00253-019-09781-4
[2] Li Y J, Ren B N, Qiao Z, et al. Characteristics of atmospheric intermediate volatility organic compounds (IVOCs) in winter and summer under different air pollution levels[J]. Atmospheric Environment, 2019, 210: 58-65. doi: 10.1016/j.atmosenv.2019.04.041
[3] Pis'ko G T, Tolstopiatova G V, Belianina T V, et al. Establishment of the maximum permissible concentrations of o-nd p-chlorotoluenes in reservoir water[J]. Gigiena i sanitariia, 1981, (2): 67-68.
[4] Sacks R, Akard M. High-speed GC analysis of VOCs-tunable selectivity and column selection. 2[J]. Environmental Science&Technology, 1994, 28(9): A428-A433.
[5] Lu G H, Li Y M, Liu J C. Toxicity Study for Halogenated Aromatics to Mixed Bacteria in River Water[J]. Procedia Environmental Sciences, 2011, 10: 209-214. doi: 10.1016/j.proenv.2011.09.036
[6] Zoeteman B, Harmsen K, Linders J, et al. Persistent organic pollutants in river water and ground water of the Netherlands[J]. Chemosphere, 1980, 9(4): 231-249. doi: 10.1016/0045-6535(80)90080-6
[7] Fatone F, Di Fabio S, Bolzonella D, et al. Fate of aromatic hydrocarbons in Italian municipal wastewater systems: An overview of wastewater treatment using conventional activated-sludge processes (CASP) and membrane bioreactors (MBRs)[J]. Water Research, 2011, 45(1): 93-104. doi: 10.1016/j.watres.2010.08.011
[8] Shin H S, Lim H H. Identification and determination of disinfection byproducts in chlorine-containing household cleansing products[J]. Chemosphere, 2017, 174: 157-164. doi: 10.1016/j.chemosphere.2017.01.090
[9] Liao R Q, Li W T, Kang Z J, et al. Distribution characteristics and ecological evaluation of chlorobenzene compounds in surface sediment of the Maowei Sea, Guangxi, China[J]. Environmental Monitoring and Assessment, 2019, 191: 309. doi: 10.1007/s10661-019-7397-0
[10] Borsdorf H, Rammler A, Schulze D, et al. Rapid on-site determination of chlorobenzene in water samples using ion mobility spectrometry[J]. Analytica Chimica Acta, 2001, 440(1): 63-70. doi: 10.1016/S0003-2670(01)01030-3
[11] Martí I, Lloret R, Martín-Alonso J, et al. Determination of chlorinated toluenes in raw and treated water samples from the Llobregat river by closed loop stripping analysis and gas chromatography-mass spectrometry detection[J]. Journal of Chromatography A, 2005, 1077(1): 68-73. doi: 10.1016/j.chroma.2005.04.051
[12] Yu S, Lee P K, Hwang S I. Groundwater contamination with volatile organic compounds in urban and industrial areas: analysis of co-occurrence and land use effects[J]. Environmental Earth Sciences, 2015, 74(4): 3661-3677. doi: 10.1007/s12665-015-4551-z
[13] Hosaini P N, Khan M F, Mustaffa N I H, et al. Concentration and source apportionment of volatile organic compounds (VOCs) in the ambient air of Kuala Lumpur, Malaysia[J]. Natural Hazards, 2017, 85(1): 437-452. doi: 10.1007/s11069-016-2575-7
[14] Eliete Z L, Soumita M, Alves V C A, et al. Distribution and sources of organic contaminants in surface sediments of Hooghly river estuary and Sundarban mangrove, eastern coast of India[J]. Marine Pollution Bulletin, 2019, 146: 39-49. doi: 10.1016/j.marpolbul.2019.05.043
[15] Zhang Z W, Pei N C, Sun Y X, et al. Halogenated organic pollutants in sediments and organisms from mangrove wetlands of the Jiulong River Estuary, South China[J]. Environmental Research, 2019, 171: 145-152. doi: 10.1016/j.envres.2019.01.028
[16] Lo I M C, Mak R K M, Lee S C H. Modified clays for waste containment and pollutant attenuation[J]. Journal of Environmental Engineering-Asce, 1997, 123(1): 25-32. doi: 10.1061/(ASCE)0733-9372(1997)123:1(25)
[17] Zhang W H, Zheng J, Zheng P P, et al. The roles of humic substances in the interactions of phenanthrene and heavy metals on the bentonite surface[J]. Journal of Soils and Sediments, 2015, 15(7): 1463-1472. doi: 10.1007/s11368-015-1112-8
[18] Wu F C, Tseng R L, Huang S C, et al. Characteristics of pseudo-second-order kinetic model for liquid-phase adsorption: A mini-review[J]. Chemical Engineering Journal, 2009, 151(1): 1-9.
[19] Deniz F. Potential use of shell biomass (Juglans regia L. ) for dye removal: Relationships between kinetic pseudo-second-order model parameters and biosorption efficiency[J]. Desalination and Water Treatment, 2014, 52(1/3): 219-226.
[20] Ho Y S. Using of "pseudo-second-order model" in adsorption[J]. Environmental Science and Pollution Research, 2014, 21(11): 7236-7237. doi: 10.1007/s11356-014-2705-2
[21] Tarasov I G, Kondylenko V P, Eremenko G M. Interparticle diffusion of aromatic hydrocarbons on aerosil surface[J]. Teoreticheskaya I Eksperimentalnaya Khimiya, 1998, 34(1): 23-26.
[22] Gorka A, Bochenek R, Warchol J, et al. Ion exchange kinetics in removal of small ions. Effect of salt concentration on inter-and intraparticle diffusion[J]. Chemical Engineering Science, 2008, 63(3): 637-650. doi: 10.1016/j.ces.2007.10.006
[23] Jiang G N, Crimi M, Fowler K, et al. Experimental design of diffusion and desorption of contaminant in heterogeneous media[J]. Water Science and Technology, 2011, 64(4): 988-998. doi: 10.2166/wst.2011.065
[24] Wu F C, Tseng R L, Juang R S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems[J]. Chemical Engineering Journal, 2009, 150(2/3): 366-373.
[25] Franguelli F P, Tannous K, Coppi C C. Biosorption of hexavalent chromium from aqueous solutions using raw coconut fiber as a natural adsorbent[J]. Chemical Engineering Communications, 2019, 206(11): 1437-1450.
[26] Shen H, Ie I R, Yuan C S, et al. Adsorption phenomenon and kinetic mechanisms of Hg-0 and HgCl2 by innovative composite sulfurized activated carbons[J]. Fuel, 2019, 256: 115894. doi: 10.1016/j.fuel.2019.115894
[27] Azizian S. Kinetic models of sorption: a theoretical analysis[J]. Journal of Colloid and Interface Science, 2004, 276(1): 47-52. doi: 10.1016/j.jcis.2004.03.048
[28] Ho Y S. Review of second-order models for adsorption systems[J]. J. Hazard Mater, 2006, 136(3): 681-689. doi: 10.1016/j.jhazmat.2005.12.043
[29] Sircar S, Myers AL. Liquid adsorption operations-equilibrium, kinetics, column dynamics, and applications[J]. Separation Science and Technology, 1986, 21(6/7): 535-562.
[30] Wu S C, Gschwend P M. Sorption kinetics of hydrophobic organic-compounds to natural sediments and soils[J]. Environmental Science&Technology, 1986, 20(7): 717-725.
[31] Sen Gupta S, Bhattacharyya K G. Kinetics of adsorption of metal ions on inorganic materials: A review[J]. Advances in Colloid and Interface Science, 2011, 162(1/2): 39-58.
[32] Saleh T A. Isotherm, kinetic, and thermodynamic studies on Hg (II) adsorption from aqueous solution by silica-multiwall carbon nanotubes[J]. Environmental Science and Pollution Research, 2015, 22(21): 16721-16731. doi: 10.1007/s11356-015-4866-z
[33] Shen J, Wang X Z, Zhang Z, et al. Adsorption and degradation of C-14-bisphenol A in a soil trench[J]. Science of the Total Environment, 2017, 607: 676-682.
[34] Kim J J, Lim S J, Ahn H, et al. Adsorption equilibria and kinetics of propane and propylene on zeolite 13X pellets[J]. Microporous and Mesoporous Materials, 2019, 274: 286-298. doi: 10.1016/j.micromeso.2018.07.039
[35] Kalinovich I, Allen-King R M, Thomas K. Distribution of carbonaceous matter in lithofacies: Impacts on HOC sorption nonlinearity[J]. Journal of Contaminant Hydrology, 2012, 133: 84-93. doi: 10.1016/j.jconhyd.2012.03.007
[36] Kang S, Jung J, Choe J K, et al. Effect of biochar particle size on hydrophobic organic compound sorption kinetics: Applicability of using representative size[J]. Science of the Total Environment, 2018, 619: 410-418.
[37] Pradhan S, Kumar P, Mehrotra I. Sorption of Aqueous Organics by Aquifer Material: Correlation of Batch Sorption Parameters with Octanol-Water Partition Coefficient[J]. Journal of Environmental Engineering, 2016, 142(8): 4016034. doi: 10.1061/(ASCE)EE.1943-7870.0001064
[38] Mallakpour S, Khadem E. Linear and nonlinear behavior of crosslinked chitosan/N-doped graphene quantum dot nanocomposite films in cadmium cation uptake[J]. Science of the Total Environment, 2019, 690: 1245-1253. doi: 10.1016/j.scitotenv.2019.06.431
[39] Awad A M, Shaikh S M R, Jalab R, et al. Adsorption of organic pollutants by natural and modified clays: A comprehensive review[J]. Separation and Purification Technology, 2019, 228: 115719.
[40] Cheng J J, Wan Q, Ge J, et al. Major factors dominating the fate of dibutyl phthalate in agricultural soils[J]. Ecotoxicology and Environmental Safety, 2019, 183: 109569-109569.
[41] Xia X H, Dai Z N, Zhang J. Sorption of phthalate acid esters on black carbon from different sources[J]. Journal of Environmental Monitoring, 2011, 13(10): 2858-2864.
[42] Kupryianchyk D, Rakowska M I, Grotenhuis J T C, et al. In situ sorption of hydrophobic organic compounds to sediment amended with activated carbon[J]. Environmental Pollution, 2012, 161: 23-29.
[43] Ncibi M C. Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis[J]. J Hazard Mater, 2008, 153(1/2): 207-212.
[44] 张凤君, 贾晗, 刘佳露, 等. 有机氯代烃在壤土中的吸附和解吸特性[J]. 吉林大学学报(地球科学版), 2015, 45(5): 1515-1522. https://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ201505023.htm
-



 下载:
下载: