Research Progress in Organosilane Modification of Clay Minerals
-
摘要:
黏土矿物由于具有独特的层状纳米结构特性,在基础研究和实际应用中引起人们普遍的关注。但黏土矿物的亲水性表面使其对材料性能的提升产生限制,因此采用有机硅烷对黏土矿物进行表面改性是改善黏土矿物表面性质的有效方法之一。经过有机硅烷改性的黏土矿物既具有黏土矿物特有的尺寸稳定性、吸附性和阻隔性等性能,又具有硅烷分子多种功能基团的反应活性,在聚合物纳米复合材料、载体材料和环境材料等领域具有广阔的应用前景,已成为近年来矿物学和材料科学的研究热点之一。综述了不同类型黏土矿物有机硅烷改性的研究进展,讨论了黏土矿物类型、有机硅烷种类和反应条件对改性黏土矿物结构的影响,同时对黏土矿物有机硅烷改性未来主要研究方向进行展望。
Abstract:Clay minerals have attracted considerable attention in basic research and practical applications due to their unique layered nanostructure. However, the hydrophilic surface of clay minerals makes it difficult to improve the properties and limits the application of materials. Therefore, the surface modification with organosilane is one of the effective methods to improve the surface properties of clay minerals. The clay minerals modified by organosilane possess dimensional stability, adsorptivity and barrier properties of clay minerals, and high reactivity of various functional groups in organosilane molecules. Therefore, the modified clay minerals have wide applications in materials science, environmental engineering and carrier materials. Accordingly, they have attracted much attention in mineralogy and material science in recent years. This paper reviewed the research progress in organosilane modification of clay minerals with different types. The influence of clay mineral type, organiosilane species and reaction conditions on the structure of modified clay minerals was discussed, and the main research directions of organosilane modification of clay minerals were prospected.
-
Key words:
- clay minerals /
- montmorillonite /
- kaolinite /
- organosilane /
- modification
-

-
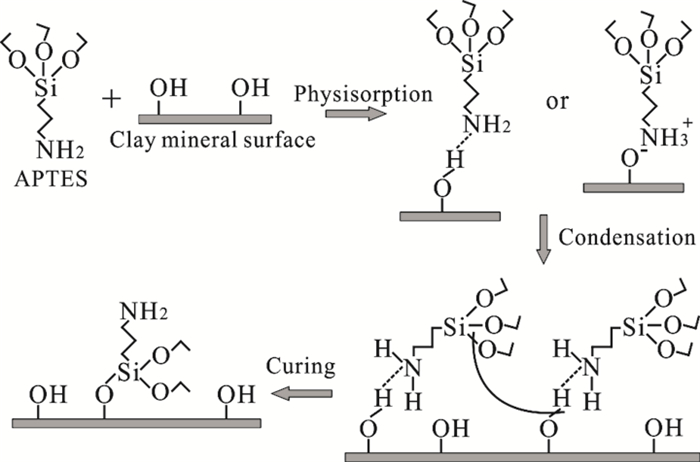
图 1 含水介质中有机硅烷嫁接机理示意图[27]
Figure 1.
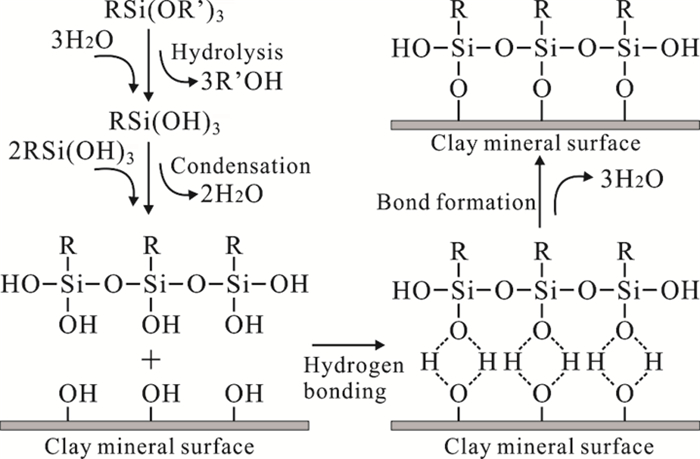
图 2 非水介质中有机硅烷嫁接机理示意图[27]
Figure 2.
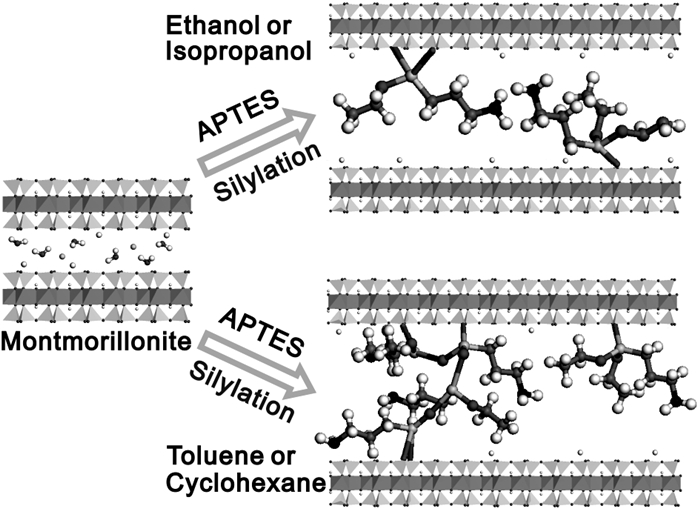
图 3 蒙脱石在不同极性溶剂中硅烷嫁接示意图[33]
Figure 3.
-
[1] Shi K, Liu Z, Yang C, et al. Novel biocompatible thermoresponsive poly(N-vinyl caprolactam)/clay nanocomposite hydrogels with macroporous structure and improved mechanical characteristics[J]. ACS applied materials & interfaces, 2017, 9(26):21979-21990. http://pubs.acs.org/doi/10.1021/acsami.7b04552
[2] Sehaqui H, Kochumalayil J, Liu A, et al. Multifunctional nanoclay hybrids of high toughness, thermal and barrier performances[J]. ACS applied materials & interfaces, 2013, 5(15):7613-7620. http://pubs.acs.org/doi/abs/10.1021/am401928d
[3] Hu P, Yang H. Polypropylene filled with kaolinite-based conductive powders[J]. Applied clay science, 2013, 83-84(5):122-128. http://www.sciencedirect.com/science/article/pii/S0169131713002688
[4] Kochumalayil JJ, Bergenstra Hle-Wohlert M, Utsel S, et al. Bioinspired and highly oriented clay nanocomposites with a xyloglucan biopolymer matrix:extending the range of mechanical and barrier properties[J]. Biomacromolecules, 2013, 14(1):84-91. doi: 10.1021/bm301382d
[5] Wang J, Cheng Q, Lin L, et al. Synergistic toughening of bioinspired poly(vinyl alcohol)-clay-nano brillar cellulose articial nacre[J]. ACS nano, 2014, 8(3):2739-2745. doi: 10.1021/nn406428n
[6] Zare Y, Rhee KY. Multistep modeling of Young's modulus in polymer/clay nanocomposites assuming the intercalation/exfoliation of clay layers and the interphase between polymer matrix and nanoparticles[J]. Composites part A:applied science and manufacturing, 2017, 102:137-144. doi: 10.1016/j.compositesa.2017.08.004
[7] He H, Ma Y, Zhu J, et al. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant conguration[J]. Applied clay science, 2010, 48(1-2):67-72. doi: 10.1016/j.clay.2009.11.024
[8] He H, Zhou Q, Martens WN, et al. Microstructure of HDTMA-modified montmorillonite and its influence on sorption characteristics[J]. Clays and clay minerals, 2006, 54(6):689-696. doi: 10.1346/CCMN.2006.0540604
[9] Huskič M, Žigon M, Ivankovič M. Comparison of the properties of clay polymer nanocomposites prepared by montmorillonite modified by silane and by quaternary ammonium salts[J]. Applied clay science, 2013, 85:109-115. doi: 10.1016/j.clay.2013.09.004
[10] Piscitelli F, Posocco P, Toth R, et al. Sodium montmorillonite silylation:unexpected effect of the aminosilane chain length[J]. Journal of colloid and interface science, 2010, 351(1):108-115. doi: 10.1016/j.jcis.2010.07.059
[11] Takahashi N, Kuroda K. Materials design of layered silicates through covalent modification of interlayer surfaces[J]. Journal of materials chemistry, 2011, 21(38):14336-14353. doi: 10.1039/c1jm10460h
[12] 杨淑勤, 袁鹏, 何宏平, 等.γ-氨丙基三乙氧基硅烷(APTES)与高岭石层间表面羟基的嫁接反应机理[J].矿物学报, 2012, 32(4):468-474. http://d.old.wanfangdata.com.cn/Periodical/kwxb201204002
[13] Khosravi H, Eslami-Farsani R. Enhanced mechanical properties of unidirectional basalt fiber/epoxy composites using silane-modified Na+-montmorillonite nanoclay[J]. Polymer testing, 2016, 55:135-142. doi: 10.1016/j.polymertesting.2016.08.011
[14] 李金梅, 黄晓玲, 苏海全.γ-氨丙基二甲基乙氧基硅烷修饰蒙脱土及硅烷化蒙脱土的性能[J].化工进展, 2014, 33(1):178-252. http://d.old.wanfangdata.com.cn/Periodical/hgjz201401031
[15] He H, Tao Q, Zhu J, et al. Silylation of clay mineral surfaces[J]. Applied Clay Science. 2013, 71(71):15-20. http://www.sciencedirect.com/science/article/pii/S016913171200258X
[16] Tao Q, Fang Y, Li T, et al. Silylation of saponite with 3-aminopropyltriethoxysilane[J]. Applied clay science, 2016, 132-133:133-139. doi: 10.1016/j.clay.2016.05.026
[17] Yuan P, Southon PD, Liu Z, et al. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane[J]. Journal of physical chemistry C, 2008, 112(40):15742-15751. doi: 10.1021/jp805657t
[18] Park A-Y, Kwon H, Woo AJ, et al. Layered double hydroxide surface modified with (3-aminopropyl)triethoxysilane by covalent bonding[J]. Advanced materials, 2005, 17(1):106-109. doi: 10.1002/(ISSN)1521-4095
[19] Ruiz-Hitzky E, Fripiatt JJ. Organomineral derivatives obtained by reacting organochlorosilanes with the surface of silicates in organic solvents[J]. Clays and clay minerals, 1976, 24(1):25-30. doi: 10.1346/CCMN
[20] Shanmugharaj AM, Rhee KY, Ryu SH. Influence of dispersing medium on grafting of aminopropyltriethoxysilane in swelling clay materials[J]. Journal of colloid and interface science, 2006, 298(2):854-859. doi: 10.1016/j.jcis.2005.12.049
[21] Voort PVD, Vansant EF. Silylation of the silica surface a review[J]. Journal of liquid chromatography & related technologies, 1996, 19(17-18):2723-2752. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/10826079608015107
[22] He H, Duchet J, Galy J, et al. Grafting of swelling clay materials with 3-aminopropyltriethoxysilane[J]. Journal of colloid and interface science, 2005, 288(1):171-176. doi: 10.1016/j.jcis.2005.02.092
[23] Shen W, He H, Zhu J, et al. Grafting of montmorillonite with different functional silanes via two different reaction systems[J]. Journal of colloid and interface science, 2007, 313(1):268-273. doi: 10.1016/j.jcis.2007.04.029
[24] Herrera NN, Letoffe J-M, Putaux J-L, et al. Aqueous dispersions of silane-functionalized laponite clay platelets. a first step toward the elaboration of water-based polymer/clay nanocomposites[J]. Langmuir, 2004, 20(5):1564-1571. doi: 10.1021/la0349267
[25] Herrera NN, Letoffe J-M, Reymond J-P, et al. Silylation of laponite clay particles with monofunctional and trifunctional vinyl alkoxysilanes[J]. Journal of materials chemistry, 2005, 15(8):863-871. doi: 10.1039/b415618h
[26] Park M, Shim I-K, Jung E-Y, et al. Modification of external surface of laponite by silane grafting[J]. Journal of physics and chemistry of solids, 2004, 65(2-3):499-501. doi: 10.1016/j.jpcs.2003.10.031
[27] Nik OG, Nohair B, Kaliaguine S. Aminosilanes grafting on FAU/EMT zeolite:effect on CO2 adsorptive properties[J]. Microporous and mesoporous materials, 2011, 143(1):221-229. doi: 10.1016/j.micromeso.2011.03.002
[28] 覃宗华, 袁鹏, 何宏平, 等.热处理蒙脱石的γ-氨丙基三乙氧基硅烷改性研究[J].矿物学报, 2012, 32:14-21. http://d.old.wanfangdata.com.cn/Periodical/kwxb201201003
[29] 沈伟, 何宏平, 朱建喜, 等.氨丙基三乙氧基硅烷嫁接蒙脱石的制备与表征[J].科学通报, 2008, 53(21):2624-2629. doi: 10.3321/j.issn:0023-074X.2008.21.014
[30] Qin Z, Yuan P, Zhu J, et al. Influences of thermal pretreatment temperature and solvent on the organosilane modification of Al13-intercalated/Al-pillared montmorillonite[J]. Applied clay science, 2010, 50(4):546-553. doi: 10.1016/j.clay.2010.10.011
[31] Sepehri S, Rafizadeh M, Hemmati M, et al. Study of the modification of montmorillonite with monofunctional and trifunctional vinyl chlorosilane[J]. Applied clay science, 2014, 97-98(4):235-240. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a5835be5901600439a96f5556777cf52
[32] Su L, Tao Q, He H, et al. Locking effect:A novel insight in the silylation of montmorillonite surfaces[J]. Materials chemistry and physics, 2012, 136(2-3):292-295. doi: 10.1016/j.matchemphys.2012.07.010
[33] Su L, Tao Q, He H, et al. Silylation of montmorillonite surfaces:dependence on solvent nature[J]. Journal of colloid and interface science, 2013, 391:16-20. doi: 10.1016/j.jcis.2012.08.077
[34] Burgentzlé D, Duchet J, Gérard JF, et al. Solvent-based nanocomposite coatings:I. dispersion of organophilic montmorillonite in organic solvents[J]. Journal of colloid and interface science, 2004, 278(1):26-39. doi: 10.1016/j.jcis.2004.05.015
[35] Song K, Sandi G. Characterization of montmorillonite surfaces after modification by organosilane[J]. Clays and clay minerals, 2001, 49(2):119-125. doi: 10.1346/CCMN
[36] Yan Z, Fu L, Yang H, et al. Amino-functionalized hierarchical porous SiO2-AlOOH composite nanosheets with enhanced adsorption performance[J]. Journal of hazardous materials, 2018, 344:1090-1100. doi: 10.1016/j.jhazmat.2017.11.058
[37] Zhang Q, Yan Z, Ouyang J, et al. Chemically modified kaolinite nanolayers for the removal of organic pollutants[J]. Applied clay science, 2018, 157:283-290. doi: 10.1016/j.clay.2018.03.009
[38] 纪阳, 刘钦甫, 杜妍娜, 等.高岭石-氨基硅烷插层复合物的制备与表征[J].硅酸盐学报, 2015, 43(4):511-518. http://d.old.wanfangdata.com.cn/Periodical/gsyxb201504023
[39] BERGAYA F, LAGALY G. Handbook of clay science:first edition[M]. Amsterdam:elsevier, 2013:707-719.
[40] Johansson U, Holmgren A, Forsling W, et al. Adsorption of silane coupling agents onto kaolinite surfaces[J]. Clay minerals, 1999, 34(2):239-246. doi: 10.1180/000985599546208
[41] Frost RL, Kristof J, Horvath E, et al. Effect of water on the formamide-intercalation of kaolinite[J]. Spectrochimica acta part A:molecular and biomolecular spectroscopy, 2000, 56(9):1711-1729. doi: 10.1016/S1386-1425(00)00224-9
[42] Murakami J, Itagaki T, Kuroda K. Synthesis of kaolinite-organic nanohybrids with butanediols[J]. Solid state ionics, 2004, 172(1-4):279-282. doi: 10.1016/j.ssi.2004.02.048
[43] Tunney JJ, Detellier C. Interlamellar covalent grafting of organic units on kaolinite[J]. Chemistry of materials, 1993, 5(6):747-748. doi: 10.1021/cm00030a002
[44] Avila LR, Faria EHD, Ciuf KJ, et al. New synthesis strategies for effective functionalization of kaolinite and saponite with silylating agents[J]. Journal of colloid and interface science, 2010, 341(1):186-193. doi: 10.1016/j.jcis.2009.08.041
[45] Tonlé IK, Diaco T, Ngameni E, et al. Nanohybrid kaolinite-based materials obtained from the interlayer grafting of 3-aminopropyltriethoxysilane and their potential use as electrochemical sensors[J]. Chemistry of materials, 2007, 19(26):6629-6636. doi: 10.1021/cm702206z
[46] Yang S, Yuan P, He H, et al. Effect of reaction temperature on grafting of γ-aminopropyl triethoxysilane (APTES) onto kaolinite[J]. Applied clay science, 2012, 62-63:8-4. doi: 10.1016/j.clay.2012.04.006
[47] Al-Harahsheh M, Shawabkeh R, Al-Harahsheh A, et al. Surface modication and characterization of Jordanian kaolinite:application for lead removal from aqueous solutions[J]. Applied surface science, 2009, 255(18):8098-8103. doi: 10.1016/j.apsusc.2009.05.024
[48] 佟景贵, 何宏平, 陶奇.分步升温法制备有机硅烷嫁接高岭石[J].硅酸盐学报, 2013, 41(11):1571-1576. http://d.old.wanfangdata.com.cn/Periodical/gsyxb201311018
[49] Kobayashi H, Matsunaga T. Amino-silane modified superparamagnetic particles with surface-immobilized enzyme[J]. Journal of colloid & interface science, 1991, 141(2):505-511. http://d.old.wanfangdata.com.cn/NSTLQK/10.1016-0021-9797(91)90347-B/
[50] Tong J, He H, Tao Q. Grafting γ-aminopropyltriethoxysilane onto the inner surface of kaolinite via 3-step thermal treatment[J]. Journal of the Chinese ceramic society, 2013, 41(11):1571-1576. http://d.old.wanfangdata.com.cn/Periodical/gsyxb201311018
[51] 苏琳娜, 陶奇, 何宏平, 等.高岭石有机硅烷嫁接及其在环氧树脂中的应用[J].高校地质学报, 2013, 19:49-50. http://d.old.wanfangdata.com.cn/Conference/7958246
[52] 张乾, 张白梅, 张玉德, 等.机械力化学法制备硅烷接枝高岭石的研究[J].硅酸盐通报, 2017, 36(3):942-946. http://d.old.wanfangdata.com.cn/Periodical/gsytb201703032
[53] Tao Q, Su L, Frost RL, et al. Silylation of mechanically ground kaolinite[J]. Clay minerals, 2014, 49(4):559-568. doi: 10.1180/claymin.2014.049.4.06
-



 下载:
下载: