Synergistic Regulation of Crystal Morphology and Particle Size of α-Hemihydrate Gypsum Under the Action of Malic Acid and Glycerol
-
摘要:
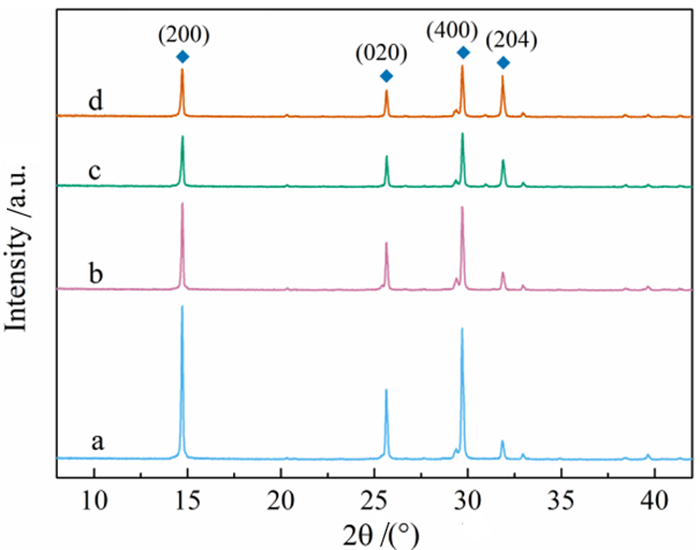
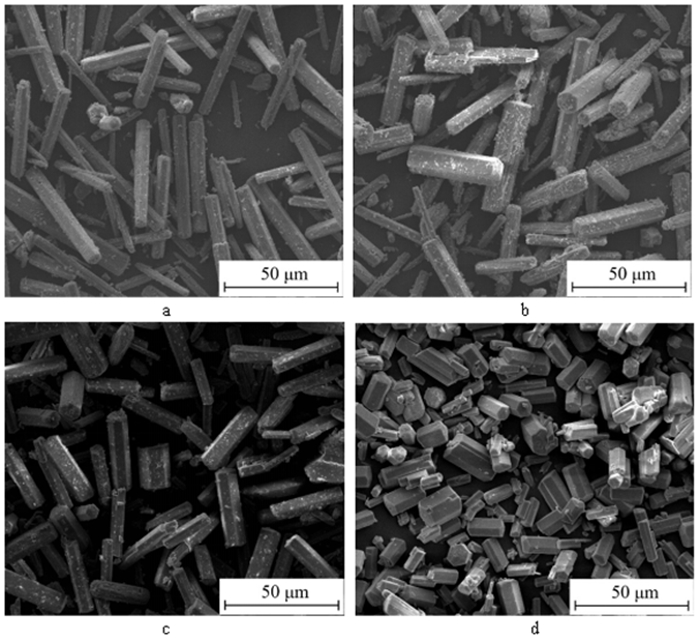
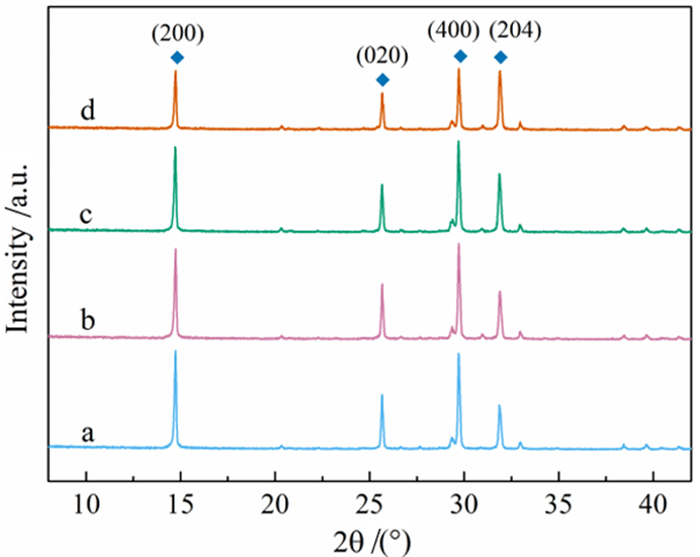
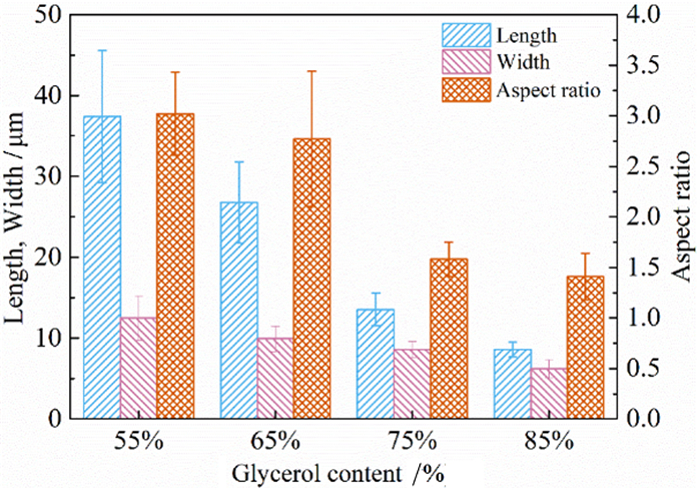
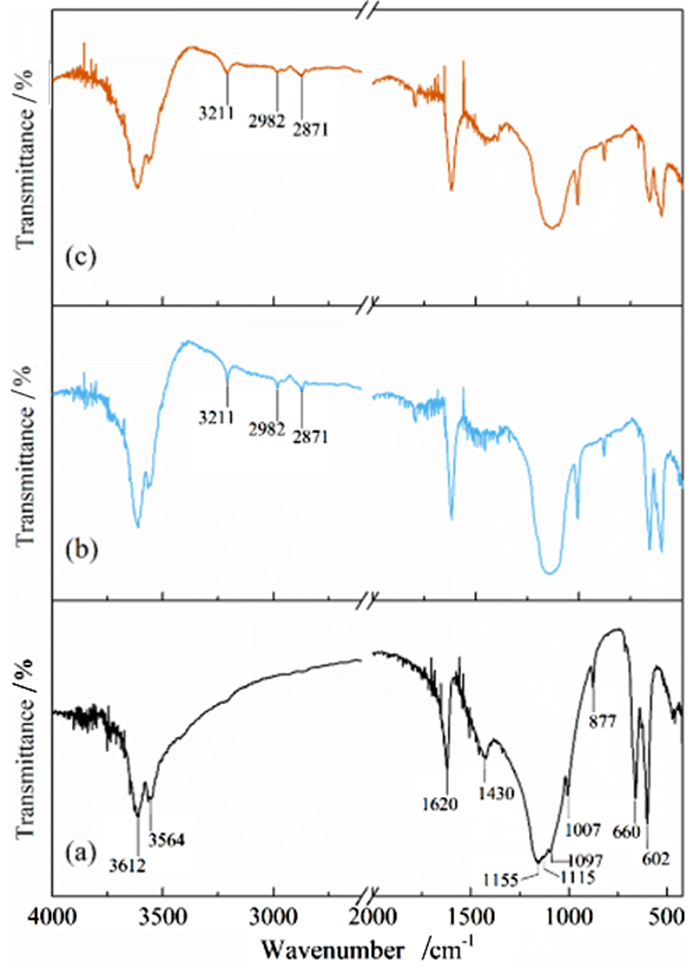
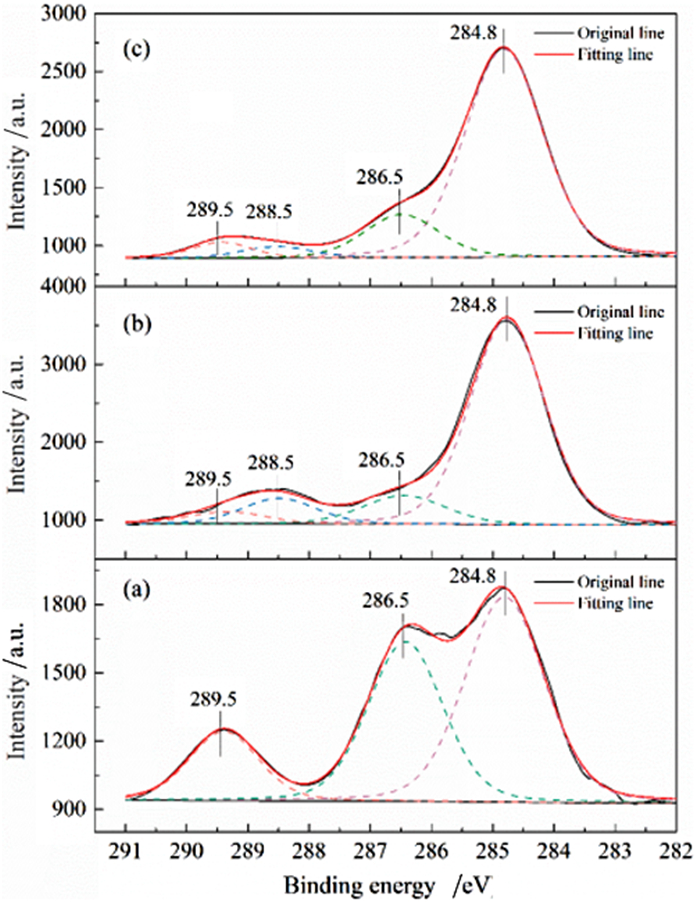
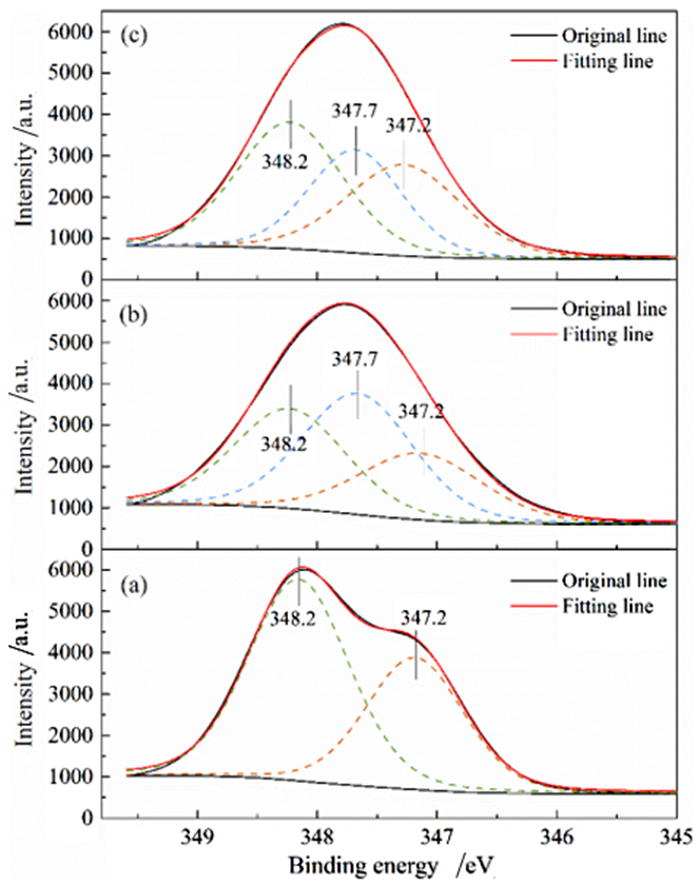
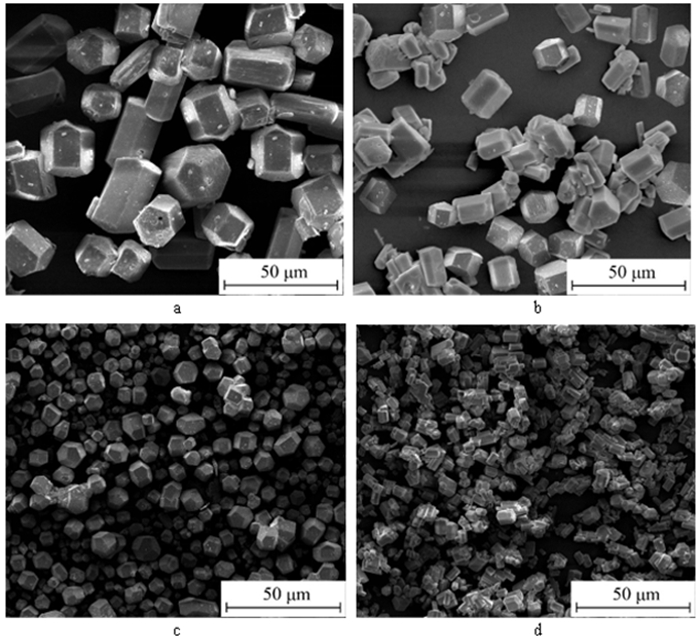
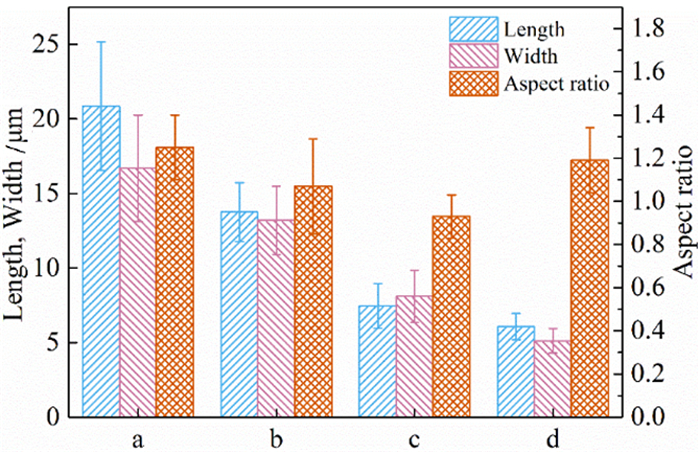
利用脱硫石膏制备高附加值的高强度α-半水石膏是拓展其利用途径的重要策略,而晶体形貌和粒度又是影响α-半水石膏品质的重要因素。以甘油水溶液为反应介质,苹果酸为媒晶剂,深入考察了苹果酸添加量和甘油浓度对晶体形貌和粒度影响,结果发现苹果酸通过与α-半水石膏晶体表面的钙质点发生络合吸附的方式主要实现对晶体形貌的调控,甘油的主要作用在于对晶体粒度的控制,其影响不是通过甘油直接在晶体表面吸附或者影响苹果酸吸附实现的。最终通过调控苹果酸添加量和甘油浓度实现了对α-半水石膏晶体形貌和粒度的协同调控并制备出具有相似形貌不同粒度的晶体。当甘油浓度为45%、苹果酸加入量为37.09×10-4 mol·kg-1时,可以生成平均粒度为18 μm左右的晶体;当甘油浓度为75%、苹果酸加入量为18.54×10-4 mol·kg-1时,可以生成平均粒度为5 μm左右的晶体。
Abstract:The preparation of high-strength α-hemihydrate gypsum with high value-added is an important way to expand the utilization. The crystal morphology and particle size of α-hemihydrate gypsum are important factors affecting its mechanical strength. Here, we investigated in deep the influence of the malic acid addition and the glycerol concentration on the crystal morphology and particle size with the reaction medium of glycerol solution and the crystal modifier of malic acid. It was found that malic acid regulated the crystal morphology by complexing adsorption with the calcium sites on the surface of α-hemihydrate gypsum crystal. The main function of glycerol was to control the crystal size, which was not achieved by glycerol adsorption directly on the surface of the crystal or affecting the adsorption of malic acid. Finally, crystal morphology and particle size of α-hemihydrate gypsum were synergistically controlled by adjusting malic acid addition and glycerol concentration, and the crystals with similar morphology and different particle size were prepared. The crystals with average size of about 18 μm were prepared with the glycerol concentration of 45% and the malic acid addition of 37.09×10-4 mol·kg-1. Meanwhile, the crystals with average size of about 5 μm were generated with the glycerol concentration of 75% and the malic acid addition of 18.54×10-4 mol·kg-1.
-
Key words:
- flue gas desulfurization gypsum /
- α-hemihydrate gypsum /
- glycerol /
- malic acid /
- particle size /
- morphology
-

-
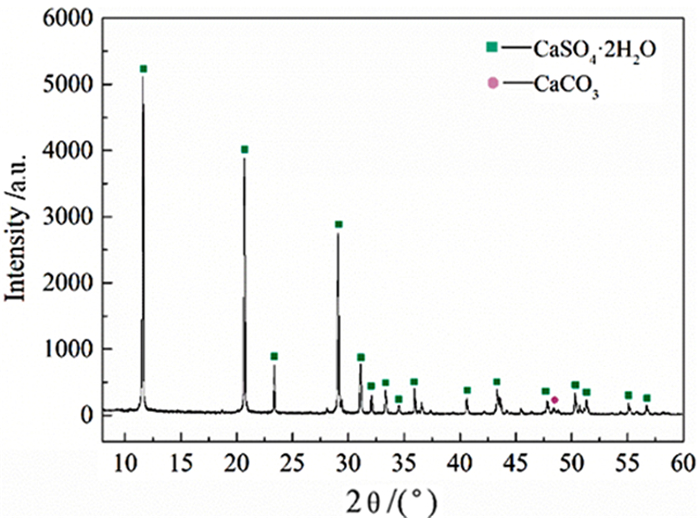
表 1 脱硫石膏的元素含量 /%
Table 1. Element content of FGD gypsum
CaO SO3 H2O SiO2 Fe2O3 Al2O3 MgO PbO K2O Others 36.21 42.30 19.62 0.65 0.32 0.25 0.10 0.06 0.03 0.46 -
[1] 白杨, 李东旭.用脱硫石膏制备高强石膏粉的转晶剂[J].硅酸盐学报, 2009, 37(7):1142-1146. doi: 10.3321/j.issn:0454-5648.2009.07.014
[2] 林敏, 万体智, 彭家惠, 等.盐溶液水热法制备α-半水脱硫石膏工艺条件研究[J].新型建筑材料, 2009, 36(6):1-3. doi: 10.3969/j.issn.1001-702X.2009.06.001
[3] Shen Zhuoxian, Guan Baohong, Fu Hailu, et al. Effect of potassium sodium tartrate and sodium citrate on the preparation of α-calcium sulfate hemihydrate from flue gas desulfurization gypsum in a concentrated electrolyte solution[J]. Journal of the American ceramic society, 2009, 92(12):2894-2899. doi: 10.1111/j.1551-2916.2009.03330.x
[4] Combe E. C., Smith D. C. Studies on the preparation of calcium sulphate hemihydrate by an autoclave process[J]. Journal of applied chemistry, 2007, 18(10):307-312. doi: 10.1002/jctb.5010181005
[5] Alfred Zürz, Ivan Odler, Felicia Thiemann, et al. Autoclave-free formation of α-hemihydrate gypsum[J]. Journal of the American ceramic society, 1991, 74(5):1117-1124. doi: 10.1111/j.1151-2916.1991.tb04351.x
[6] Kostic-Pulek Aleksandra, Marinkovic Slobodanka, Popov Svetlana, et al. The treatment of gypsum as a product of the flue gas desulphurization process[J]. Ceram. Silikaty, 2005, 49(2):115-119.
[7] Guan Baohong, Yang Liuchun, Wu Zhongbiao, et al. Preparation of α-calcium sulfate hemihydrate from FGD gypsum in K, Mg-containing concentrated CaCl2 solution under mild conditions[J]. Fuel, 2009, 88(7):1286-1293. doi: 10.1016/j.fuel.2009.01.004
[8] Guan Baohong, Jiang Guangming, Fu Hailu, et al. Thermodynamic preparation window of alpha calcium sulfate hemihydrate from calcium sulfate dihydrate in non-electrolyte glycerol-water solution under mild conditions[J]. Industrial and engineering chemistry research, 2011, 50(23):13561-13567. doi: 10.1021/ie201040y
[9] Guan Baohong, Jiang Guangming, Wu Zhongbiao, et al. Preparation of α-calcium sulfate hemihydrate from calcium sulfate dihydrate in methanol-water solution under mild conditions[J]. Journal of the American ceramic society, 2011, 94(10):3261-3266. doi: 10.1111/j.1551-2916.2011.04470.x
[10] Jiang Guangming, Fu Hailu, Savino Keith, et al. Nonlattice cation-SO42- ion pairs in calcium sulfate hemihydrate nucleation[J]. Crystal growth and design, 2017, 13(13):5128-5134. https://ceramics.onlinelibrary.wiley.com/doi/abs/10.1111/j.1551-2916.2011.04470.x
[11] Zhu Bao Lin, Huang Xin, Guo Ye. Influence of cement particle size distribution on strength of hardened cement paste[J]. Key engineering materials, 2011, 477(477):118-124.
[12] 彭家惠, 陈明凤, 张建新, 等.有机酸结构对α-半水脱硫石膏晶体形貌的影响及其调晶机理[J].四川大学学报(工程科学版), 2012, 44(1):166-172.
[13] Wang Peng, Lee Eun Jung, Park Chee Sung, et al. Calcium sulfate hemihydrate powders with a controlled morphology for use as bone cement[J]. Journal of the American ceramic society, 2008, 91(6):2039-2042. doi: 10.1111/j.1551-2916.2008.02358.x
[14] Chen Qiaoshan, Jiang Guangming, Jia Caiyun, et al. A facile method to control the structure and morphology of α-calcium sulfate hemihydrate[J]. Crystengcomm, 2015, 17(44):8549-8554. doi: 10.1039/C5CE01923K
[15] Jiang Guangming, Li Junxi, Nie Yunliang, et al. Immobilizing water into crystal lattice of calcium sulfate for its separation from water-in-oil emulsion[J]. Environmental science and technology, 2016, 50(14):7650-7657. doi: 10.1021/acs.est.6b01152
[16] Kong B., Guan B., Yates M. Z., Wu Z. Control of α-calcium sulfate hemihydrate morphology using reverse microemulsions[J]. Langmuir, 2012, 28(40):14137-14142. doi: 10.1021/la302459z
[17] Mao Xiulong, Song Xingfu, Lu Guimin, Sun Yuzhu, Xu Yanxia, Yu Jianguo. Control of crystal morphology and size of calcium sulfate whiskers in aqueous HCl solutions by additives:experimental and molecular dynamics simulation studies[J]. Industrial and engineering chemistry research, 2015, 54(17):4781-4787. doi: 10.1021/acs.iecr.5b00585
[18] Painter Paul C, Snyder Randy W, Starsinic Michael, et al. Concerning the application of FT-IR to the study of coal:a critical assessment of band assignments and the application of spectral analysis programs[J]. Applied spectroscopy, 1981, 35(5):475-485. doi: 10.1366/0003702814732256
[19] Zhao Wenpeng, Wu Yumin, Xu Jun, et al. Retracted article:effect of ethylene glycol on hydrothermal formation of calcium sulfate hemihydrate whiskers with high aspect ratios[J]. RSC advances, 2015, 5(62):50544-50548. doi: 10.1039/C5RA07712E
[20] Morris M. D., Berger A, Mahadevan-Jansen A, Infrared and raman spectroscopy[M], Marcel dekker Inc., 1976.
[21] Mielczarski J. A., Cases J. M., Alnot M., et al. XPS characterization of chalcopyrite, tetrahedrite, and tennantite surface products after different conditioning. 1. aqueous solution at pH 10[J]. Langmuir, 1996, 12(10):455-479.
[22] Ikumapayi Fatai, Makitalo Maria, Johansson Bjorn, Rao Kota Hanumantha. Recycling of process water in sulphide flotation:Effect of calcium and sulphate ions on flotation of galena[J]. Minerals engineering, 2012, 39(12):77-88.
[23] Deng Meijiao, Karpuzov Dimitre, Liu Qingxia, et al. Cryo-XPS study of xanthate adsorption on pyrite[J]. Surface and interface analysis, 2013, 45(4):805-810. doi: 10.1002/sia.5165
[24] Fairthorne G., Fornasiero D., Ralston J., et al. Formation of a copper-butyl ethoxycarbonyl thiourea complex[J]. Analytica chimica acta, 1997, 346(2):237-248. doi: 10.1016/S0003-2670(97)00111-6
[25] Landis W. J, Martin J. R. X-ray photoelectron spectroscopy applied to gold-decorated mineral standards of biological interest[J]. Journal of vacuum science and technology A, 1984, 2(2):1108-1111. doi: 10.1116/1.572680
[26] Huo S. J., He J. M., Chen L. H., et al. Adsorption configuration of sodium 2-quinoxalinecarboxylate on iron substrate:Investigation by in situ SERS, XPS and theoretical calculation[J]. Spectrochim. Acta A, 2015, 156:123-130.
[27] Demri B., Muster D. XPS study of some calcium compounds[J]. Journal of materials processing technology, 1995, 55(3):311-314.
[28] Guan Qingjun, Sun Wei, Hu Yuehua, et al. Simultaneous control of particle size and morphology of α-CaSO4·1/2H2O with organic additives[J]. Journal of the American ceramic society, 2019, 102(5):2440-2450.
[29] Guan Qingjun, Hu Yuehua, Tang Honghu, et al. Preparation of α-CaSO4·1/2H2O with tunable morphology from flue gas desulphurization gypsum using malic acid as modifier:A theoretical and experimental study[J]. Journal of colloid and interface science, 2018, 530:292-301. doi: 10.1016/j.jcis.2018.06.068
[30] Guan Qingjun, Tang Honghu, Sun Wei, et al. Insight into influence of glycerol on preparing α-CaSO4·1/2H2O from flue gas desulfurization gypsum in glycerol-water solutions with succinic acid and NaCl[J]. Industrial and engineering chemistry research, 2017, 56:9831-9838. doi: 10.1021/acs.iecr.7b02067
-



 下载:
下载: