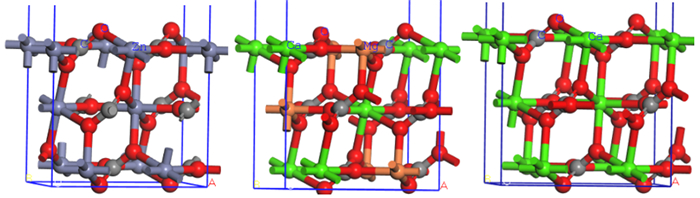
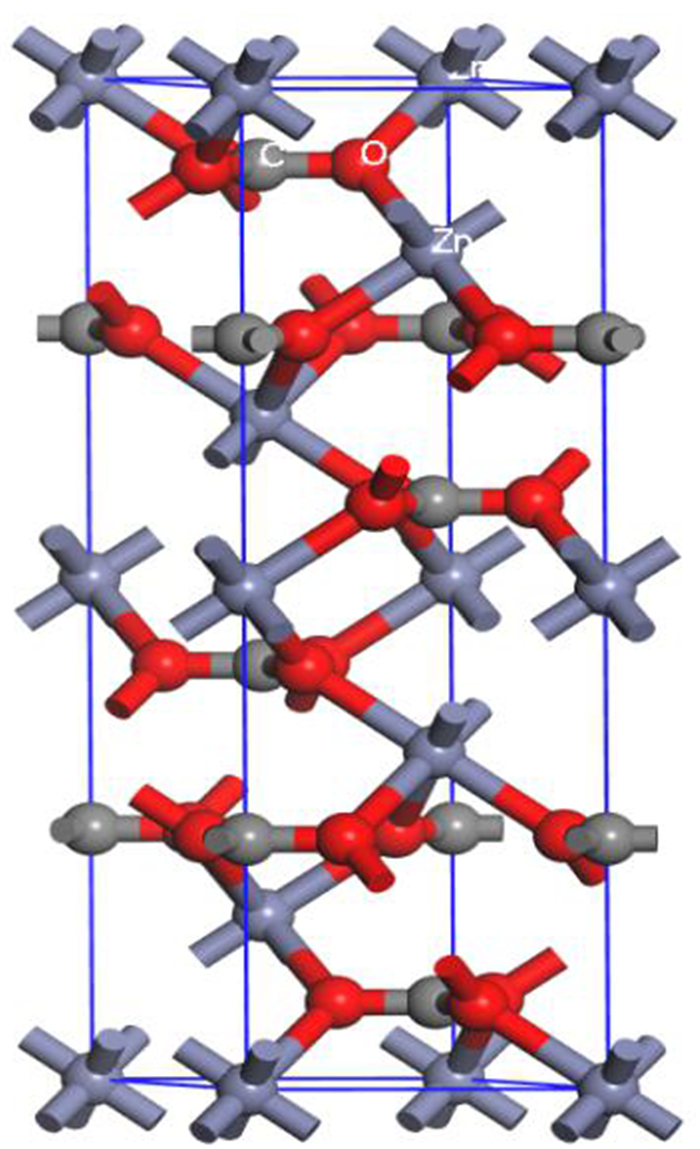
Research on Crystal Anisotropy and Surface Properties of Smithsonite
-
摘要:
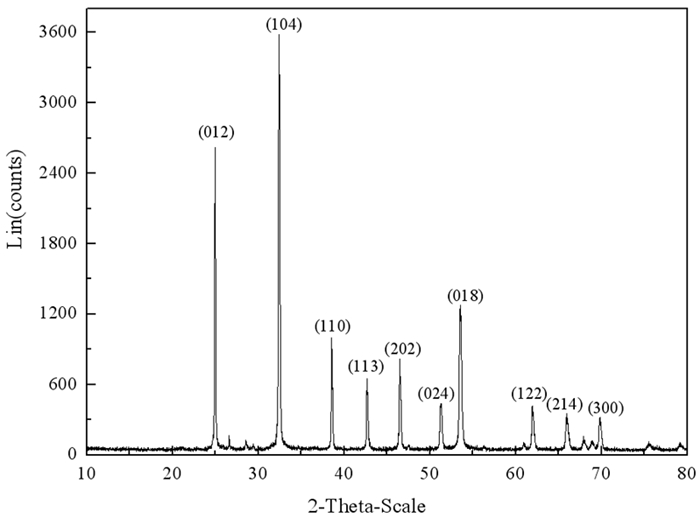
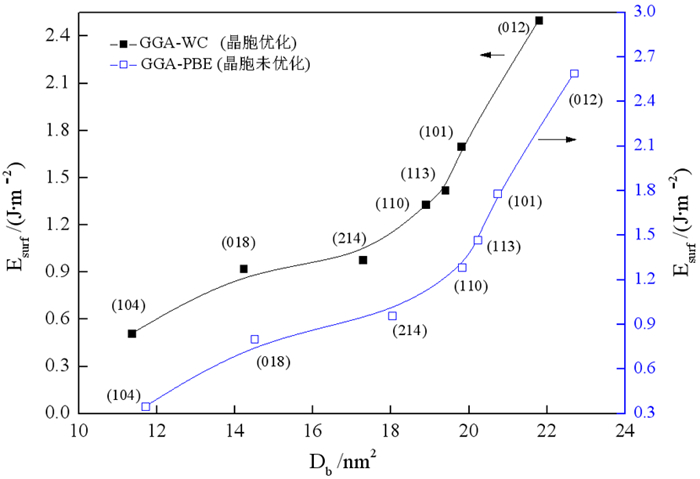
通过密度泛函理论和断裂键理论,研究了菱锌矿化学键特性和断裂键各向异性,确定了常见解理面。结果表明,菱锌矿晶体中Zn-O键强度低于C-O键,解理时优先发生Zn-O键断裂。菱锌矿各晶面只有Zn-O断裂时,断裂键密度与表面能正相关,但当C-O键和Zn-O键不均等断裂时断裂键密度与表面能不存在对应关系。表面能和断裂键密度证明(104)面、(018)面和(214)面是菱锌矿最常见解理面,且(018)面和(214)面Zn位点活性大于(104)面。分析还得出菱锌矿和钙镁碳酸盐脉石矿物(方解石、白云石)常见解理面活性位点空间排布规律相似,表面金属位点的化学性质差异是实现三种矿物分选、筛选高效药剂的重要依据。
Abstract:On basis of density functional theory and population of broken bond theory, the chemical bond properties and anisotropic surface broken bonds were studied, and the common cleavage planes was determined. The results showed that the strength of Zn-O bond is lower than that of C-O bond, and the broken of Zn-O bond occurs firstly during the minerals cleavage. The broken bond population is positively correlated with the surface energy when only Zn-O bond broken on each crystal plane, but there is no corresponding relationship between the broken bond population and the surface energy when both C-O and Zn-O broken occur at the same time.The surface energy and broken bond population indicted that (104), (018) and (214) surfaces are the most common surfaces for smithsonite. The activity of Zn site at (018) and (214) surfaces is greater than that at (104) surfaces. The analysis also showed that the active sites on the surface of calcite and dolomite are arranged in the same space, and the chemical properties of the metal sites on the surface are the important factors that determined the mineral separation and efficient agent screening.
-
Key words:
- smithsonite /
- anisotropic /
- broken bond /
- cleavage /
- surface properties
-

-
表 1 菱锌矿中Mulliken键布局分析
Table 1. Mulliken bond population of smithsonit
键类型 最小键布局 最大键布局 键的平均布局 最小键长/nm 最大键长/nm 平均键长/nm Zn-O 0.18 0.27 0.22 0.208 0.227 0.213 C-O 0.80 0.96 0.87 0.128 0.133 0.131 表 2 菱锌矿不同晶面的断裂键种类、断裂键密度和表面能
Table 2. Broken bond types, density and surface energy of different crystal planes of smithsonite
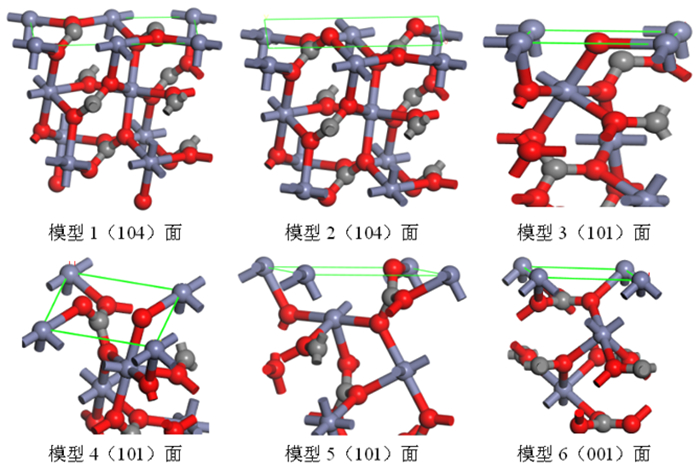
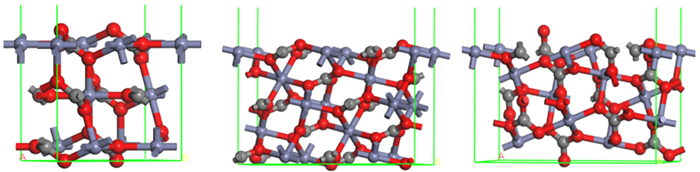
晶面 模型序号 断裂键类型 Db/nm2 Esurf(J/m2) Zn-O C-O (104) 1 4 2 17.12 4.299 2 4 0 11.36 0.511 3 3 2 19.80 4.589 (101) 4 3 1 15.84 2.346 5 5 0 19.80 1.697 (001) 6 3 0 15.79 1.692 表 3 菱锌矿、白云石和方解石(104)面金属位点性质
Table 3. Character of metal sites on the (104) plane of smithsonite, dolomite and calcite
矿物 位点种类 相邻位点间距/nm 位点密度/nm-2 电负性 酸分类 菱锌矿 Zn 0.468 5.68 1.6 交界酸 白云石 Mg 0.485 2.64 1.2 硬酸 Ca 0.485 2.64 1.0 硬酸 方解石 Ca 0.499 5.05 1.0 硬酸 -
[1] 李胜荣. 结晶学与矿物学[M]. 北京: 地质出版社, 2010.
[2] GOUVEIA A F, FERRER M M, et al. Modeling the atomicscale structure, stability and morphological transformations in the tetragonal phase of LaVO4[J]. Chemical Physics Letters, 2016, 660: 87-92. doi: 10.1016/j.cplett.2016.08.013
[3] GAO ZY, SUN W, HU YH. Mineral cleavage nature and surface energy: Anisotropic surface broken bonds consideration[J]. Trans. Nonferrous Met. Soc. China, 2014, 24: 2930-2937. doi: 10.1016/S1003-6326(14)63428-2
[4] 高志勇. 三种含钙矿物晶体各向异性与浮选行为关系的基础研究[D]. 长沙: 中南大学, 2013.
[5] 朱一民, 张洋洋, 南楠, 等. 磷灰石晶体及表面基因的第一性原理计算[J]. 金属矿山, 2020(6): 87-93. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS202006015.htm
[6] 沈智豪, 张谦, 方健, 等. 菱锌矿表面硫化研究进展[J]. 有色金属(选矿部分), 2021(1): 37-46, 59.
[7] HAN C, LI TT, ZHANG W, et al. Density functional theory study on the surface properties and floatability of hemimorphite and smithsonite[J]. Minerals, 2018, 8(12): 1-13. http://www.researchgate.net/publication/329146854_Density_Functional_Theory_Study_on_the_Surface_Properties_and_Floatability_of_Hemimorphite_and_Smithsonite
[8] LIU J, ZENG Y, EJTEMAEI M., et al. DFT simulation of S-species interaction with smithsonite (001) surface: Effect of water moleculeadsorption position[J]. Results in Physics, 2019, 15: 102575. doi: 10.1016/j.rinp.2019.102575
[9] 王瑜, 刘建, 曾勇, 等. 不同硫组分在菱锌矿表面吸附的计算研究[J]. 有色金属工程, 2019, 9(6): 69-75, 83. https://www.cnki.com.cn/Article/CJFDTOTAL-YOUS201906012.htm
[10] CUI WY, CHEN JH, LI YQ, et al. Interactions of xanthate molecule with different mineral surfaces: A comparative study of Fe, Pband Zn sulfide and oxide minerals with coordination chemistry[J]. Minerals Engineering, 2020, 159: 106565. doi: 10.1016/j.mineng.2020.106565
[11] ZHAO WC, LIU DW, FENG QC, et al. DFT insights into the electronic properties and adsorption mechanism of HS on smithsonite (101) surface[J]. Minerals Engineering, 2019, 141: 105846. doi: 10.1016/j.mineng.2019.105846
[12] LIU WP, WANG ZX, WANG XM, et al. Smithsonite flotation with lauryl phosphate[J]. Minerals Engineering, 2020, 147: 106155. doi: 10.1016/j.mineng.2019.106155
[13] LIU M, CHEN JH, CHEN Y, et al. Interaction between smithsonite and carboxyl collectors with different molecular structure in the presence of water: A theoretical and experimental study[J]. Applied Surface Science, 2020, 510: 145410. doi: 10.1016/j.apsusc.2020.145410
[14] 高志勇, 孙伟, 刘晓文, 等. 白钨矿和方解石晶面的断裂键差异及其对矿物解理性质和表面性质的影响[J]. 矿物学报, 2010, 30(4): 470-475. https://www.cnki.com.cn/Article/CJFDTOTAL-KWXB201004012.htm
[15] BAI JZ, WANG JZ, YIN WZ, et al. Influence of sodium phosphate salts with different chain length on the flotation behavior of magnesite and dolomite[J]. Minerals, 2020, 10(11): 1031. doi: 10.3390/min10111031
[16] WANG L, HU GY, SUN W, et al. Selective flotation of smithsonite from dolomite by using novel mixed collector system[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(5): 1082-1089. doi: 10.1016/S1003-6326(19)65016-8
-



 下载:
下载: