Alkali-Modified Zeolite: Adsorption Performance for Pb and Ammonia-Nitrogen and Its Remediation Effect on Soil from Rare Earth Mines
-
摘要:
针对废弃离子型稀土矿山中的重金属铅和氨氮复合污染问题,本文采用木醋液、氢氧化钠、木醋液-氢氧化钠对天然沸石进行改性,利用扫描电镜(SEM)、比表面积测定(BET)、X射线衍射(XRD)分析改性前后沸石的微观结构和物相组成变化。开展室内模拟溶液中吸附动力实验;以现场采集的土壤为基质,进行柱淋滤实验及土壤中稳定化实验,分析了天然沸石和氯化钠、氢氧化钠、木醋液-氢氧化钠三种不同改性沸石对铅和氨氮的形态影响。结果表明:碱改性沸石和碱+木醋液改性沸石对200mg/L铅的去除率超过94%,对30mg/L氨氮的去除率大于65%;2%(质量百分比)为碱改性沸石的最佳添加比例,使土壤中铅有效态固化率达52%,氨氮由不稳定态向稳定态转化。现场中试实验证明,添加修复材料6个月后,碱改性沸石吸附稳定土壤中氨氮达94.61%。碱改性沸石不仅制备工艺简单,价格便宜,无二次污染,而且对土壤中铅和氨氮复合污染有很好的稳定化效果,可作为用于废弃稀土矿山土壤修复稳定化材料之一。
Abstract:BACKGROUND Co-contaminated soils in rare earth mining areas, particularly with Pb and ammonia-nitrogen, present a significant environmental challenge. These contaminants have the potential for lasting, irreversible effects on both ecosystems and human health. Therefore, developing efficient, sustainable, and cost-effective soil remediation techniques is critical. Remediation in these areas is not only vital for reducing environmental pollution and protecting ecosystems but also supports sustainable mining practices and resource utilization. Current research in this field, especially regarding Pb and ammonia-nitrogen co-contamination, is limited. Zeolite adsorption, a popular method globally, is effective in treating heavy metal contamination in soils, showing superior results over lime and phosphate treatments. However, enhancing the adsorption capacity of natural zeolite is necessary, for which various modification methods are being explored. Among these, wood vinegar, a product of biomass pyrolysis, shows promise in improving pollutant removal due to its antimicrobial properties. This study explores the potential of wood vinegar as an additive to alkali-modified zeolite to stabilize heavy metals and ammonia-nitrogen in soils.
OBJECTIVES In order to tackle the remediation of co-contaminated soil in rare earth mines.
METHODS Wood vinegar, sodium hydroxide, and wood vinegar-sodium hydroxide were employed for zeolite modification, and Pb and ammonia-nitrogen speciation were determined by a continuous extraction method. Dynamic adsorption experiments were conducted to preliminarily analyze distinct modified zeolites’ adsorption performance. Optimal mixing ratio of modified zeolites with soil samples was determined by column leaching experiments. Through indoor stabilization experiments, the stabilization effects of different modified zeolites on Pb and ammonia-nitrogen were compared, and chemical speciation changes and their environmental implications were discussed. Investigating the stabilizing impact of various modified zeolites on soil Pb and ammonia-nitrogen, including their influence on specific ammonia-nitrogen forms, was further explored. SEM, BET, and XRD analyses were employed to assess morphological changes and phase composition variations of zeolites before and after modification. Considering process and cost, alkali-modified zeolite was chosen for pilot-scale tests, verifying the stabilization efficacy of remediation materials in practical applications.
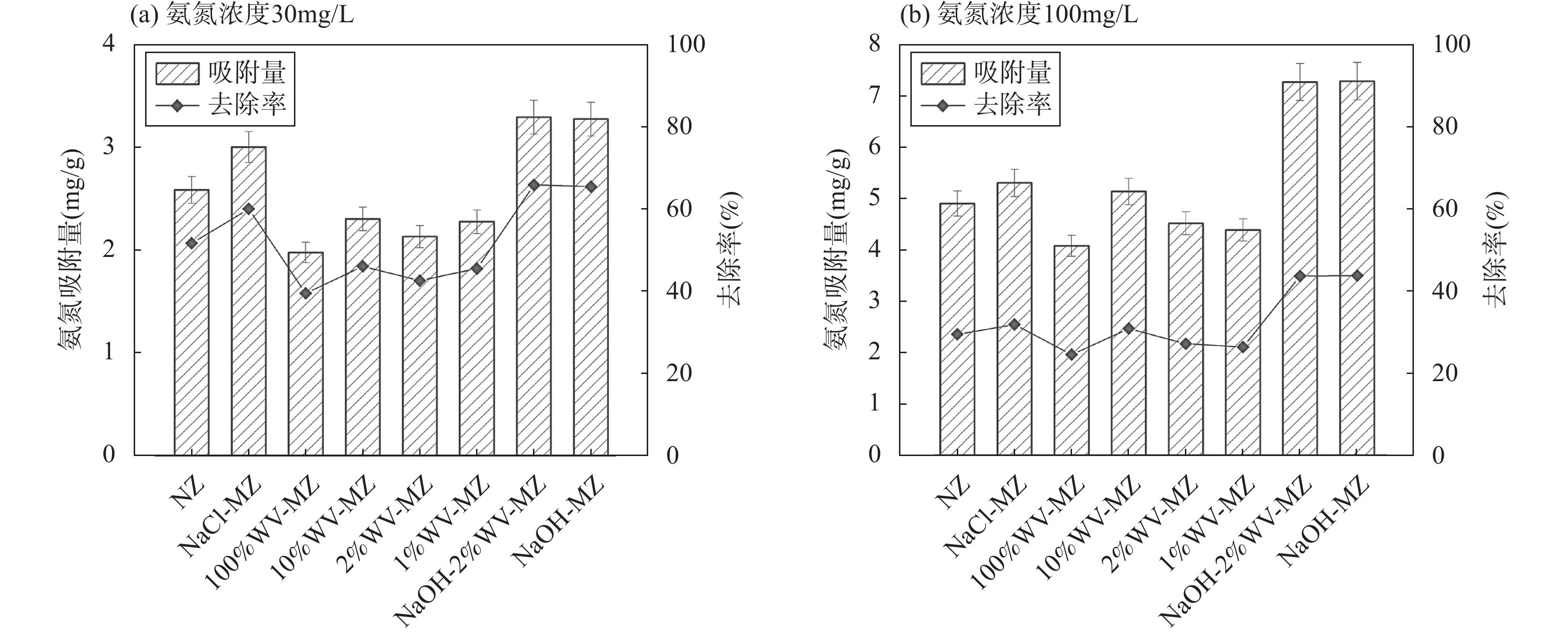
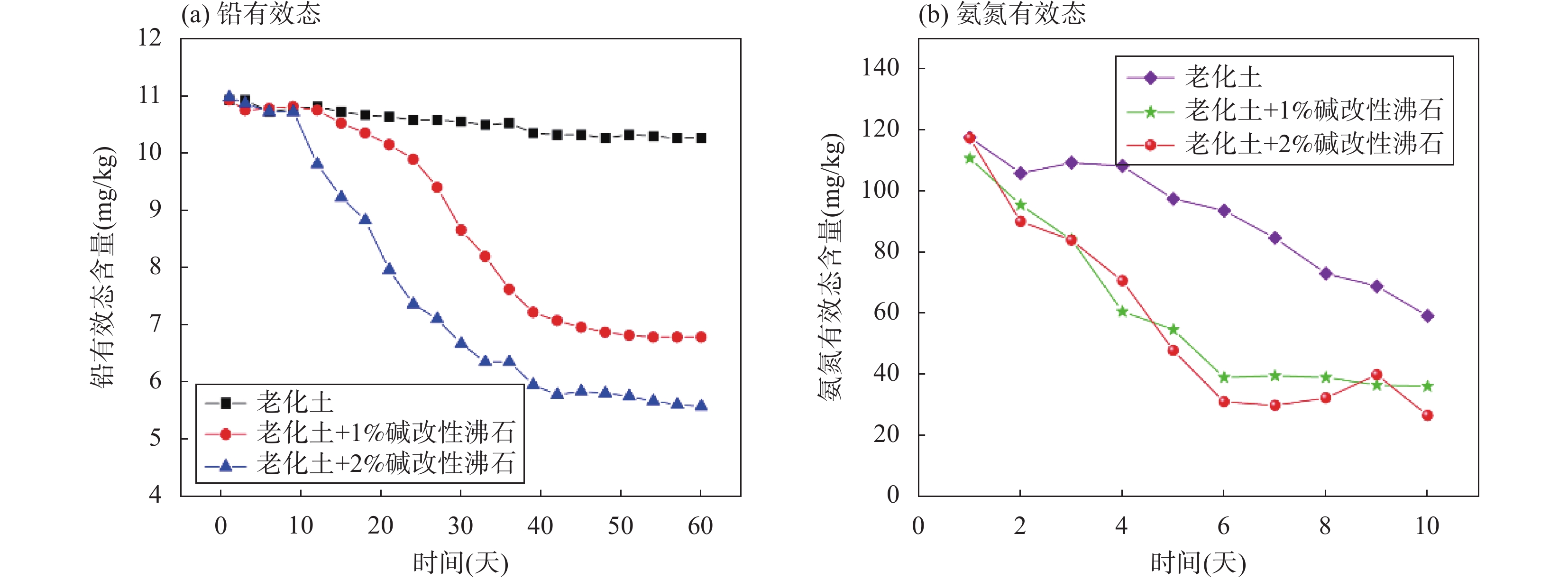
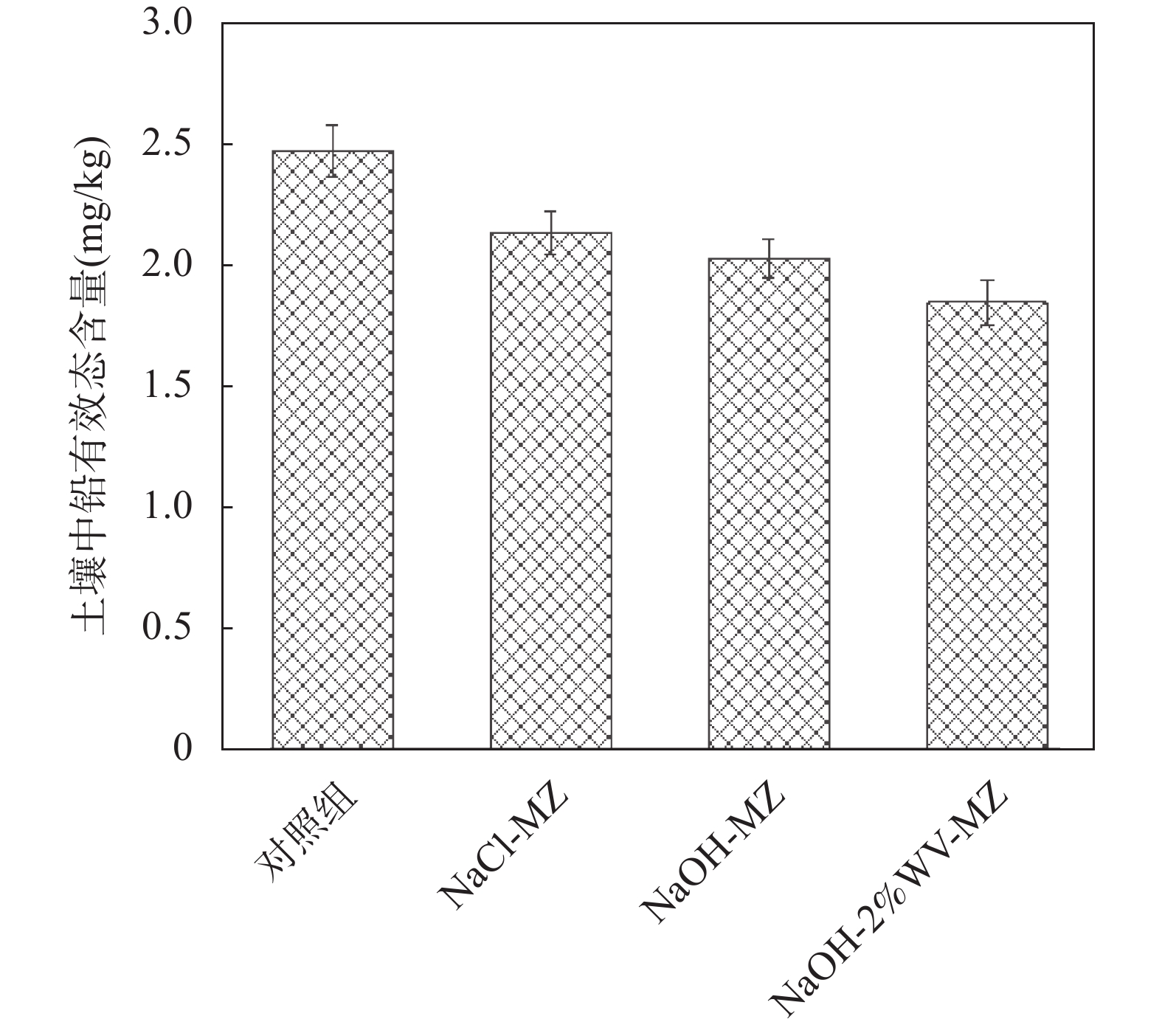
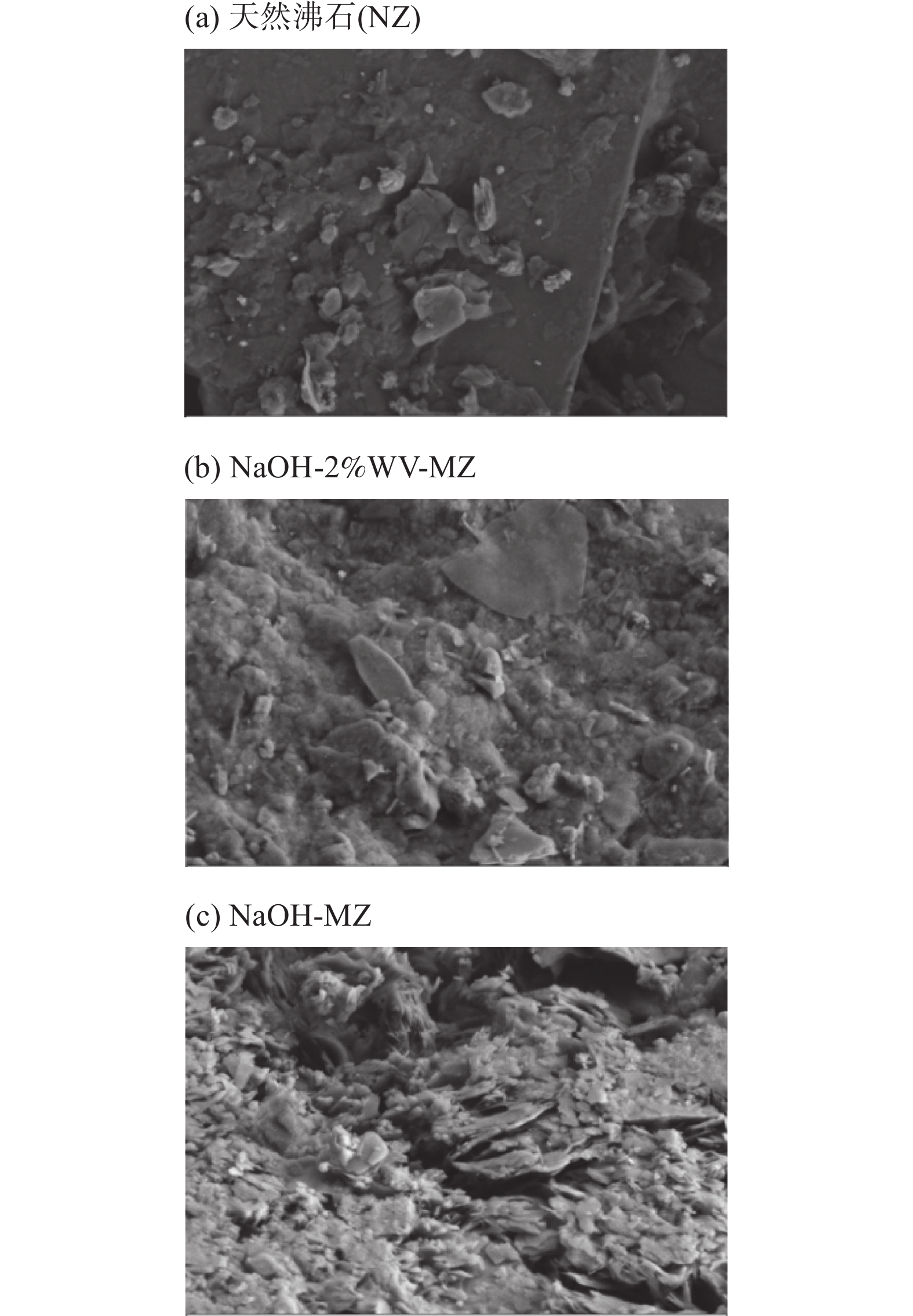
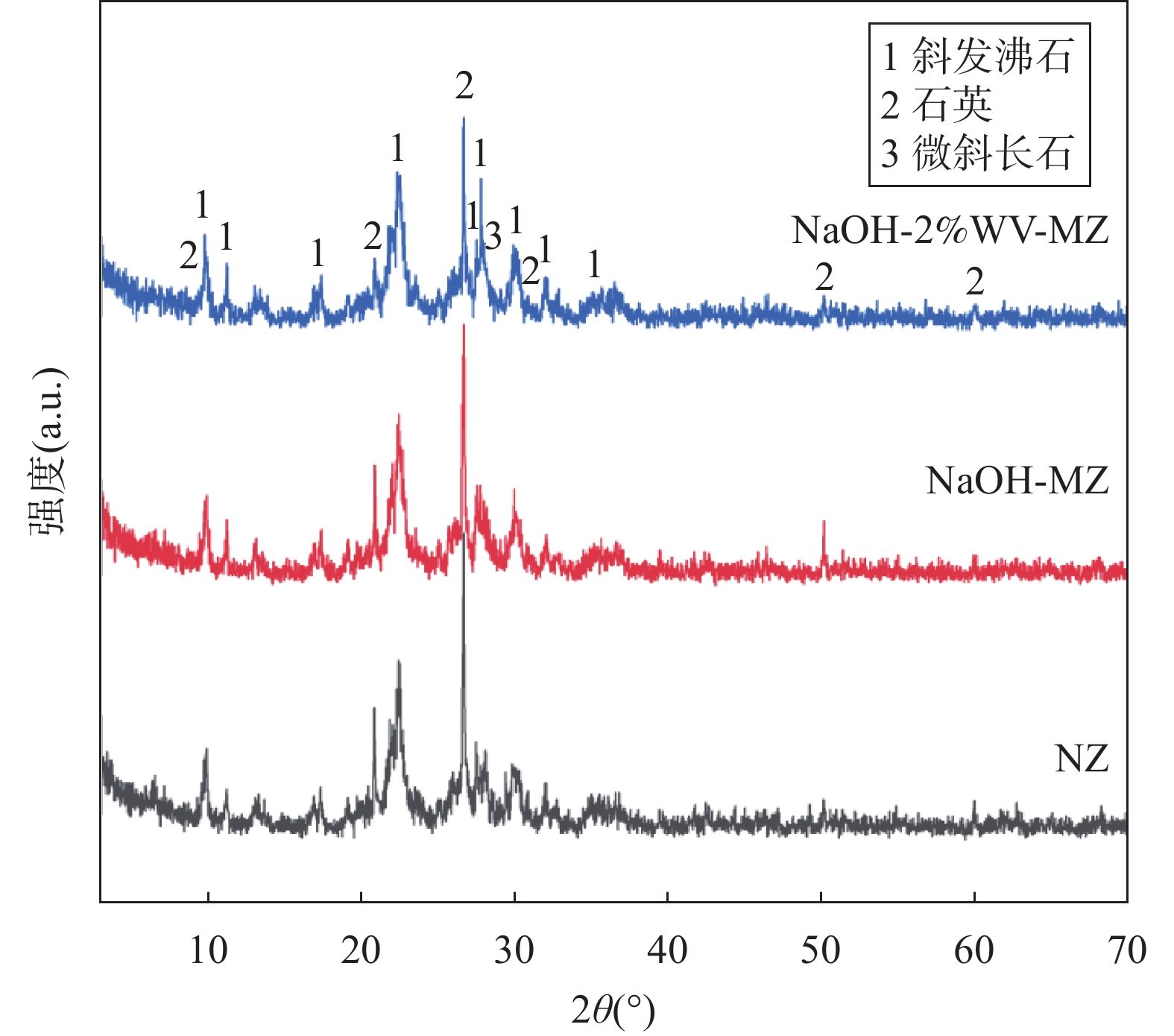
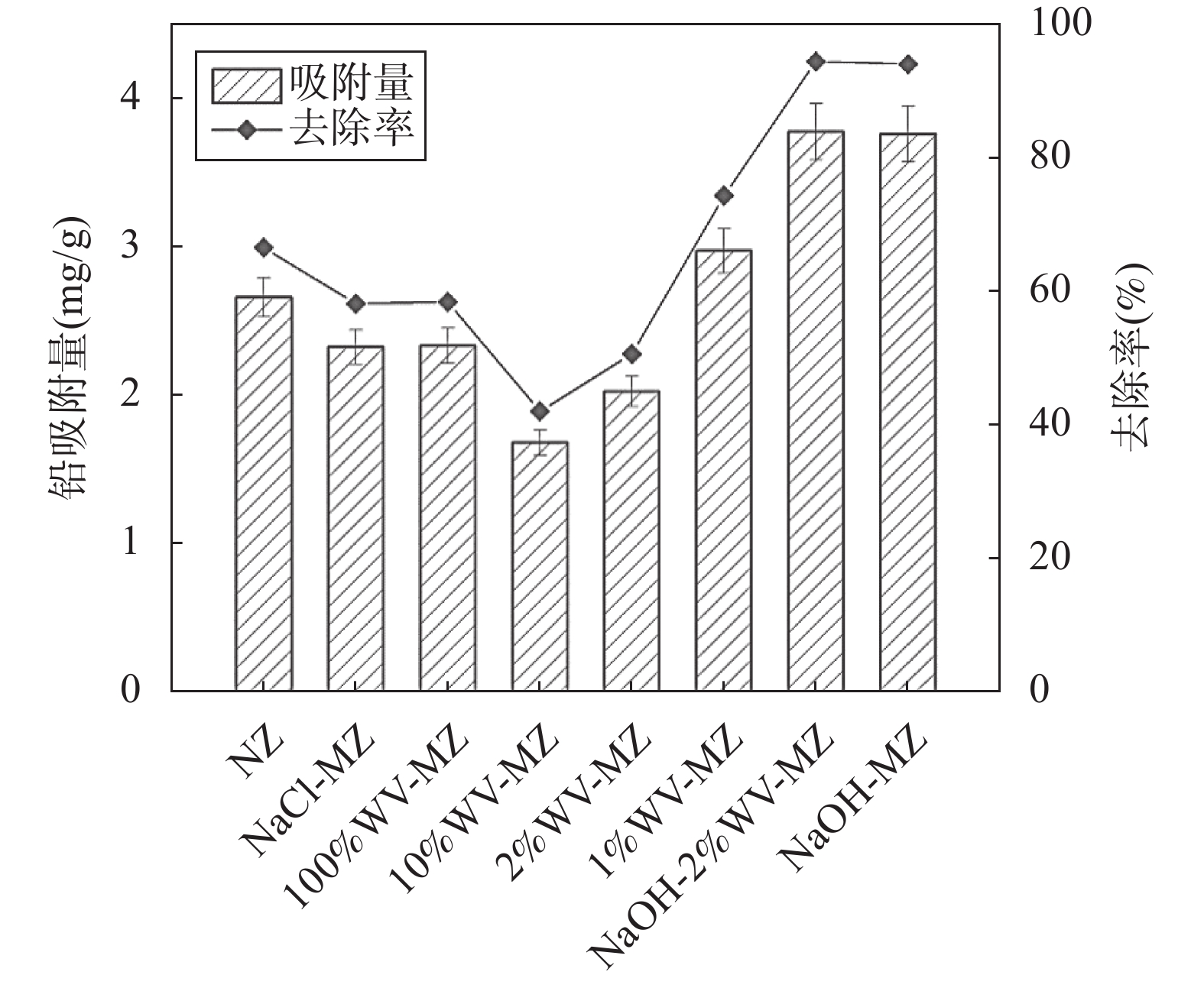
RESULTS Adsorption performance in aqueous solutions. NaOH-MZ (modified zeolite by sodium hydroxide) and NaOH-2%WV-MZ (modified zeolite by sodium hydroxide combined with 2% wood vinegar) exhibited outstanding performance in Pb removal, achieving over 94% removal from a 200mg/L initial concentration. For ammonia-nitrogen removal, NaOH-2%WV-MZ and NaOH-MZ outperformed other zeolites, with removal rates of 66% and 65% for 30 mg/L, and 44% for 100mg/L. The adsorption efficiency of modified zeolite on ammonia-nitrogen varied with concentration, suggesting a correlation with zeolite dosage. Increasing modified zeolite dosage can enhance adsorption efficiency, as higher concentrations of ammonia-nitrogen reach saturation more rapidly. Column leaching experiments. At a 2% addition ratio of alkali-modified zeolite in soil, the most effective reduction in available Pb and ammonia-nitrogen forms was observed. Reduction trends continued over time, with a 50% decrease in Pb available form after 40 days and a 73% decrease in ammonia-nitrogen’s available form after 6 days. Stabilization effects on soil Pb and ammonia-nitrogen. NaOH-MZ and NaOH-2%WV-MZ exhibited stabilization effects on soil Pb and facilitated the transformation of ammonia-nitrogen. After 7 days, NaOH-MZ reduced available Pb content by 20%, and NaOH-2%WV-MZ by 26%, surpassing control and NaCl-MZ. The stabilization effect persisted over time, with optimal outcomes observed in the 6th week for 2% NaOH-MZ and NaOH-2%WV-MZ. Microscopic analysis. Alkali-modified zeolites showed structural changes favoring adsorption, with NaOH-2%WV modification leading to a looser structure with enhanced adsorption potential. The alkaline modification process involves cation exchange and leaching of silica components, transforming impurities like quartz into active silicates and amorphous silica. Pilot-scale tests. In an abandoned rare earth mining area, pilot-scale tests demonstrated the efficacy of 2% alkali-modified zeolite in reducing soil ammonia-nitrogen content. After 6 months, a remarkable reduction of 94.61% was achieved, highlighting the potential of alkali-modified zeolites for sustainable soil remediation.
CONCLUSIONS Alkali and alkali-wood vinegar modifications enhanced zeolite structure, reducing impurities like quartz, and improving adsorption. Experiments show that alkali-modified zeolites, particularly at a 2% addition rate, effectively remove Pb and ammonia-nitrogen, achieving up to 50% Pb stabilization and a 94.61% reduction in ammonia-nitrogen in field trials. This research informs effective soil remediation technologies and sustainable development. While showing promise, further investigation is needed to assess long-term stability.
-
Key words:
- modified zeolite /
- ionic rare earth mine /
- available Pb /
- ammonia nitrogen form /
- soil remediation
-

-
表 1 模拟雨水成分
Table 1. The composition of the simulative rainwater.
成分指标 浓度
(mg/L)成分指标 浓度
(mg/L)Na+ 0.12 HCO3 − 14.7 K+ 0.07 Cl− 5.26 Ca2+ 0.13 SO4 2− 1.17 Mg2+ 0.03 pH 5.69 表 2 土壤中氨氮形态的萃取方法
Table 2. Extraction method of ammonia nitrogen form in soil.
萃取剂 氨氮形态 萃取次数
(次)平衡时间
(h)75%(V∶V)乙醇 残渣态 5 0.3 去离子水 水溶态 5 3 1mol/L氯化钾溶液 可交换态 3 3 表 3 不同沸石对土壤中铅有效态的影响(2%添加比例)
Table 3. Effect of different zeolites (2%) on Pb effective status in soil.
稳定化时间
(周)铅有效态含量(mg/kg) NZ NaCl-MZ NaOH-MZ NaOH-2%WV-MZ 1 12.39 11.35 10.81 10.76 2 12.32 10.64 10.10 9.20 3 11.64 9.47 6.52 6.16 4 11.55 8.00 6.39 6.06 5 10.85 7.42 6.27 6.00 6 9.63 6.52 6.11 5.86 固化率(%) 24 49 52 59 表 4 改性沸石的比表面积和孔容变化
Table 4. Specific surface area and pore volume variation of the modified zeolites.
样品 比表面积
(m²/g)孔容
(cm3/g)NZ 30.381 0.015 NaOH-MZ 29.979 0.014 NaOH-2%WV-MZ 28.291 0.014 表 5 中试实验土壤中氨氮的含量
Table 5. The content of ammonia nitrogen in the experimental soil.
实验组 不同时间的氨氮含量(mg/kg) 0个月 2个月 6个月 CK 336.583 220.583 57.308 T3 294.383 31.717 15.867 -
[1] 赵晋,王海超,陈春丽. 废弃稀土矿山的环境修复方案[J]. 有色冶金设计与研究, 2018, 39(5): 8−11. doi: 10.3969/j.issn.1004-4345.2018.05.003
Zhao J,Wang H C,Chen C L. Environmental remediation scheme of abandoned rare earth mines[J]. Nonferrous Metallurgy Design and Research, 2018, 39(5): 8−11. doi: 10.3969/j.issn.1004-4345.2018.05.003
[2] Wang Y Y,Wang G F,Sun M Q,et al. Environmental risk assessment of the potential “Chemical Time Bomb” of ion-adsorption type rare earth elements in urban areas[J]. The Science of the Total Environment, 2022, 822: 153305. doi: 10.1016/j.scitotenv.2022.153305
[3] 张塞,于扬,王登红,等. 赣南离子吸附型稀土矿区土壤重金属形态分布特征及生态风险评价[J]. 岩矿测试, 2020, 39(5): 726−738. doi: 10.15898/j.cnki.11-2131/td.201911050152
Zhang S,Yu Y,Wang D H,et al. Soil morphological distribution characteristics and ecological risk assessment of heavy metals in ion-adsorbed rare earth mining area in Southern Jiangxi[J]. Rock and Mineral Analysis, 2020, 39(5): 726−738. doi: 10.15898/j.cnki.11-2131/td.201911050152
[4] 刘斯文,黄园英,赵文博,等. 赣南北部黄陂河流域离子型稀土矿地区水质与健康风险评价[J]. 岩矿测试, 2022, 41(3): 488−498. doi: 10.3969/j.issn.0254-5357.2022.3.ykcs202203014
Liu S W,Huang Y Y,Zhao W B,et al. Water quality and health risk assessment in ionic rare earth mine area in Huangpi River Basin in Northern Gannan[J]. Rock and Mineral Analysis, 2022, 41(3): 488−498. doi: 10.3969/j.issn.0254-5357.2022.3.ykcs202203014
[5] 谭启海,赵永红,黄璐,等. 硫酸铵对离子型稀土矿区土壤重金属的释放和形态转化影响[J]. 有色金属科学与工程, 2022, 13(6): 134−144. doi: 10.13264/j.cnki.ysjskx.2022.06.018
Tan Q H,Zhao Y H,Huang L,et al. Effect of ammonium sulfate on heavy metal release and morphological transformation in soil of ionic rare earth mineral areas[J]. Nonferrous Metals Science and Engineering, 2022, 13(6): 134−144. doi: 10.13264/j.cnki.ysjskx.2022.06.018
[6] 李宇,梁音,曹龙熹,等. 离子型稀土矿区小流域氨氮污染物地表迁移特征[J]. 土壤, 2021, 53(6): 1271−1280.
Li Y,Liang Y,Cao L X,et al. Surface migration characteristics of ammonia nitrogen pollutants in small watersheds of ionic rare earth mining areas[J]. Soil, 2021, 53(6): 1271−1280.
[7] 张培,谢海云,曹广祝,等. 硫酸铵浸出离子型稀土矿对土壤和地下水污染的研究现状[J]. 矿冶, 2021, 30(4): 95−101,110.
Zhang P,Xie H Y,Cao G Z,et al. Research status of soil and groundwater pollution caused by ammonium sulfate leaching of ionic rare earth minerals[J]. Mining and Metallurgy, 2021, 30(4): 95−101,110.
[8] 刘晖,俞莹,吴火亮,等. 江西稀土矿山土壤修复与复绿的研究进展[J]. 南方林业科学, 2020, 48(6): 74−78.
Liu H,Yu Y,Wu H L,et al. Research progress on soil remediation and greening in rare earth mines in Jiangxi[J]. Southern Forestry Science, 2020, 48(6): 74−78.
[9] 张诚,张启军,黄彬. 离子型稀土矿开采污染防治[J]. 有色冶金节能, 2021, 37(6): 46−49. doi: 10.19610/j.cnki.cn11-4011/tf.2021.06.010
Zhang C,Zhang Q J,Huang B. Pollution prevention and control of ionic rare earth mining[J]. Energy Conservation in Nonferrous Metallurgy, 2021, 37(6): 46−49. doi: 10.19610/j.cnki.cn11-4011/tf.2021.06.010
[10] 杜超,于凤娟,曾婉秋,等. 离子型稀土矿区河流氨氮污染及防治对策研究[J]. 赣南师范大学学报, 2022, 43(6): 101−107.
Du C,Yu F J,Zeng W Q,et al. Research on river ammonia nitrogen pollution and prevention measures in ionic rare earth mining areas[J]. Journal of Gannan Normal University, 2022, 43(6): 101−107.
[11] 郭钟群,赵奎,金解放,等. 离子型稀土矿环境风险评估及污染治理研究进展[J]. 稀土, 2019, 40(3): 115−126.
Guo Z Q,Zhao K,Jin J F,et al. Progress in environmental risk assessment and pollution control of ionic rare earth mines[J]. Rare Earth, 2019, 40(3): 115−126.
[12] Garau G,Castaldi P,Santona L,et al. Influence of red mud,zeolite and lime on heavy metal immobilization,culturable heterotrophic microbial populations and enzyme activities in a contaminated soil[J]. Geoderma, 2007, 142(1-2): 47−57. doi: 10.1016/j.geoderma.2007.07.011
[13] 杨岳,吴涛涛,王闰民,等. 沸石改性及对水中氨氮的吸附性能研究[J]. 环境与发展, 2020, 32(9): 118−120.
Yang Y,Wu T T,Wang R M,et al. Study on the modification of zeolite and its adsorption performance for ammonia nitrogen in water[J]. Environment and Development, 2020, 32(9): 118−120.
[14] 王喆,谭科艳,梁明会,等. 天然丝光沸石表面重构改性及其在水中去除重金属的应用[J]. 岩矿测试, 2020, 32(9): 118−120. doi: 10.15898/j.cnki.11-2131/td.201802110018
Wang Z,Tan K Y,Liang M H,et al. Surface reconstruction and modification of natural silk photozeolite and its application of heavy metal removal in water[J]. Rock and Mineral Analysis, 2020, 32(9): 118−120. doi: 10.15898/j.cnki.11-2131/td.201802110018
[15] 王冠,方战强,成文,等. 改性沸石对诺氟沙星的吸附行为及机理[J]. 华南师范大学学报(自然科学版), 2018, 50(3): 41−50. doi: 10.6054/j.jscnun.2018040
Wang G,Fang Z Q,Cheng W,et al. Adsorption behavior and mechanism of modified zeolite on norfloxacin[J]. Journal of South China Normal University (Natural Science Edition), 2018, 50(3): 41−50. doi: 10.6054/j.jscnun.2018040
[16] 马志军,李冰川. 阜新天然沸石改性除氟试验研究[J]. 硅酸盐通报, 2014, 33(3): 676−681.
Ma Z J,Li B C. Experimental study on fluoride removal by modifying natural zeolite in Fuxin[J]. Silicate Bulletin, 2014, 33(3): 676−681.
[17] 张曦,吴为中,温东辉,等. 氨氮在天然沸石上的吸附及解吸[J]. 环境化学, 2003, 22(2): 166−171.
Zhang X,Wu W Z,Wen D H,et al. Adsorption and desorption of ammonia nitrogen on natural zeolite[J]. Environmental Chemistry, 2003, 22(2): 166−171.
[18] 张宏华,柏晓云,林建伟,等. 天然沸石和铁改性方解石联合覆盖对底泥铜和铅释放的控制效果及机制[J]. 环境化学, 2022, 41(8): 2729−2741.
Zhang H H,Bai X Y,Lin J W,et al. Control effect and mechanism of combined coverage of natural zeolite and iron modified calcite on copper and Pb release from sediment[J]. Environmental Chemistry, 2022, 41(8): 2729−2741.
[19] 秦余丽,熊仕娟,徐卫红,等. 不同镉浓度及pH条件下纳米沸石对土壤镉形态及大白菜镉吸收的影响[J]. 环境科学, 2016, 37(10): 4030−4043.
Qin Y L,Xiong S J,Xu W H,et al. The effect of nanozeolite on soil cadmium forms and cadmium absorption in Chinese cabbage under different cadmium concentrations and pH conditions[J]. Environmental Science, 2016, 37(10): 4030−4043.
[20] Wang Y F,Lin F,Pang W Q. Ion exchange of ammonium in natural and synthesized zeolites[J]. Journal of Hazardous Materials, 2008, 160(2-3): 371−375. doi: 10.1016/j.jhazmat.2008.03.006
[21] Caputo D,Pepe F. Experiments and data processing of ion exchange equilibria involving Italian natural zeolites:A review[J]. Microporous and Mesoporous Materials, 2007, 105(3): 222−231. doi: 10.1016/j.micromeso.2007.04.024
[22] 汤泉,陈南春. 天然沸石改性方法的研究进展[J]. 材料导报, 2009, 23(S1): 439−441.
Tang Q,Chen N C. Research progress on modification methods of natural zeolite[J]. Materials Introduction, 2009, 23(S1): 439−441.
[23] 赵启文,张兴儒,严刚. 斜发沸石对锌冶炼废水中钙镁离子的吸附研究[J]. 无机盐工业, 2010, 42(3): 45−47.
Zhao Q W,Zhang X R,Yan G. Study on the adsorption of calcium and magnesium ions in zinc smelting wastewater by clinoptilolite[J]. Inorganic Salt Industry, 2010, 42(3): 45−47.
[24] 王代芝,刘娟敏. 酸碱改性沸石处理低浓度含Cd2+废水的研究[J]. 中国非金属矿工业导刊, 2015(2): 16−18.
Wang D Z,Liu J M. Research on the treatment of low concentration Cd2+ wastewater with acid-base modified zeolite[J]. China Journal of Nonmetallic Mineral Industry, 2015(2): 16−18.
[25] Masrournia M. Evaluation of heavy metal removal from wastewater using Iranain modified natural clinoptilolite[J]. Asian Journal of Chemistry, 2013, 25(7): 3867−3870. doi: 10.14233/ajchem.2013.13827
[26] 左思敏,荆肇乾,陶梦妮. 天然沸石和改性沸石在废水处理中的应用研究[J]. 应用化工, 2019, 48(5): 1136−1139.
Zuo S M,Jing Z Q,Tao M N. Research on the application of natural zeolite and modified zeolite in wastewater treatment[J]. Applied Chemistry, 2019, 48(5): 1136−1139.
[27] 李艺,史会剑,吴春辉. 阳离子表面活性剂改性沸石吸附水体中重金属的研究综述[J]. 净水技术, 2020, 39(12): 73−79.
Li Y,Shi H J,Wu C H. Research review on the adsorption of heavy metals in water by cationic surfactant modified zeolites[J]. Water Purification Technology, 2020, 39(12): 73−79.
[28] Ghader M,Payam Z,Ali T,et al. From microporous to mesoporous mineral frameworks:An alliance between zeolite and chitosan[J]. Carbohydrate Research, 2020, 489: 107930. doi: 10.1016/j.carres.2020.107930
[29] 罗宁临,李忠武,黄梅,等. 壳聚糖(改性)-沸石对农田土壤重金属镉钝化技术研究[J]. 湖南大学学报(自然科学版), 2020, 47(4): 132−140.
Luo N L,Li Z W,Huang M,et al. Study on the passivation technology of heavy metal cadmium in farmland soil using chitosan (modified) zeolite[J]. Journal of Hunan University (Natural Science Edition), 2020, 47(4): 132−140.
[30] Gao L,Zhang C Y,Sun Y,et al. Effect and mechanism of modification treatment on ammonium and phosphate removal by ferric-modified zeolite[J]. Environmental Technology, 2019, 40: 1959−1968. doi: 10.1080/09593330.2018.1435729
[31] 佟小薇,朱义年. 沸石改性及其去除水中氨氮的实验研究[J]. 环境工程学报, 2009, 3(4): 635−638.
Tong X W,Zhu Y N. Experimental study on zeolite modification and its removal of ammonia nitrogen from water[J]. Journal of Environmental Engineering, 2009, 3(4): 635−638.
[32] Chen Y X,Huang X D,Han Z Y,et al. Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting[J]. Chemosphere, 2010, 78(9): 1177−1181. doi: 10.1016/j.chemosphere.2009.12.029
[33] Liu L,Guo X P,Wang S Q,et al. Effects of wood vinegar on properties and mechanism of heavy metal competitive adsorption on secondary fermentation based composts[J]. Ecotoxicology and Environmental Safety, 2018, 150: 270−279. doi: 10.1016/j.ecoenv.2017.12.037
[34] Li Z C,Wu L J,Sun S,et al. Disinfection and removal performance for Escherichia coli,toxic heavy metals and arsenic by wood vinegar-modified zeolite[J]. Ecotoxicology and Environmental Safety, 2019, 174: 129−136. doi: 10.1016/j.ecoenv.2019.01.124
[35] Jia W,Yi Y J,Zeng M M. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction[J]. Journal of Environmental Management, 2016, 178: 63−69. doi: 10.1016/j.jenvman.2016.04.046
-



 下载:
下载: