Hydrochemical origins and weathering-controlled CO2 consumption rates in the mainstream of the Yangtze River
-
摘要:
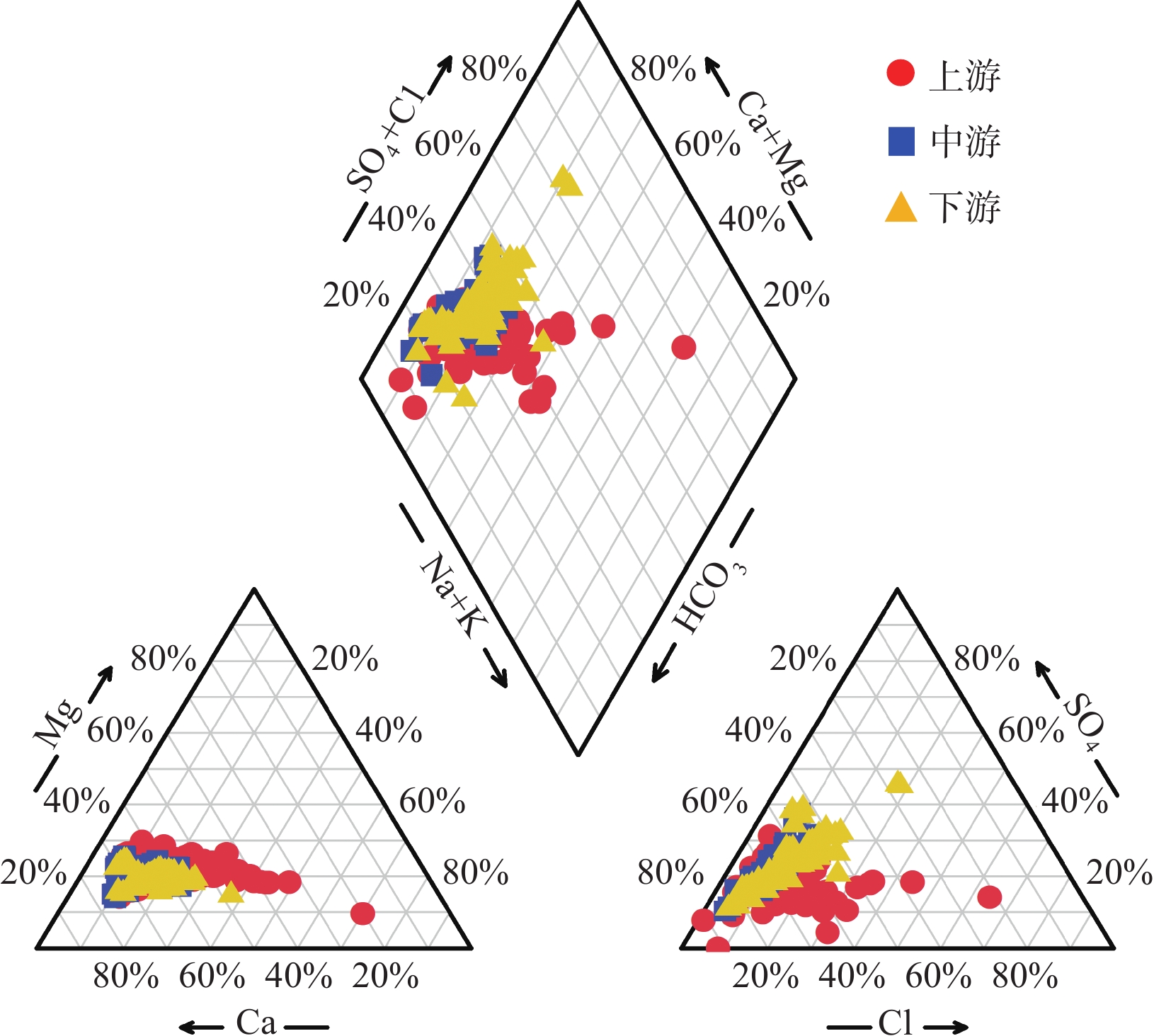
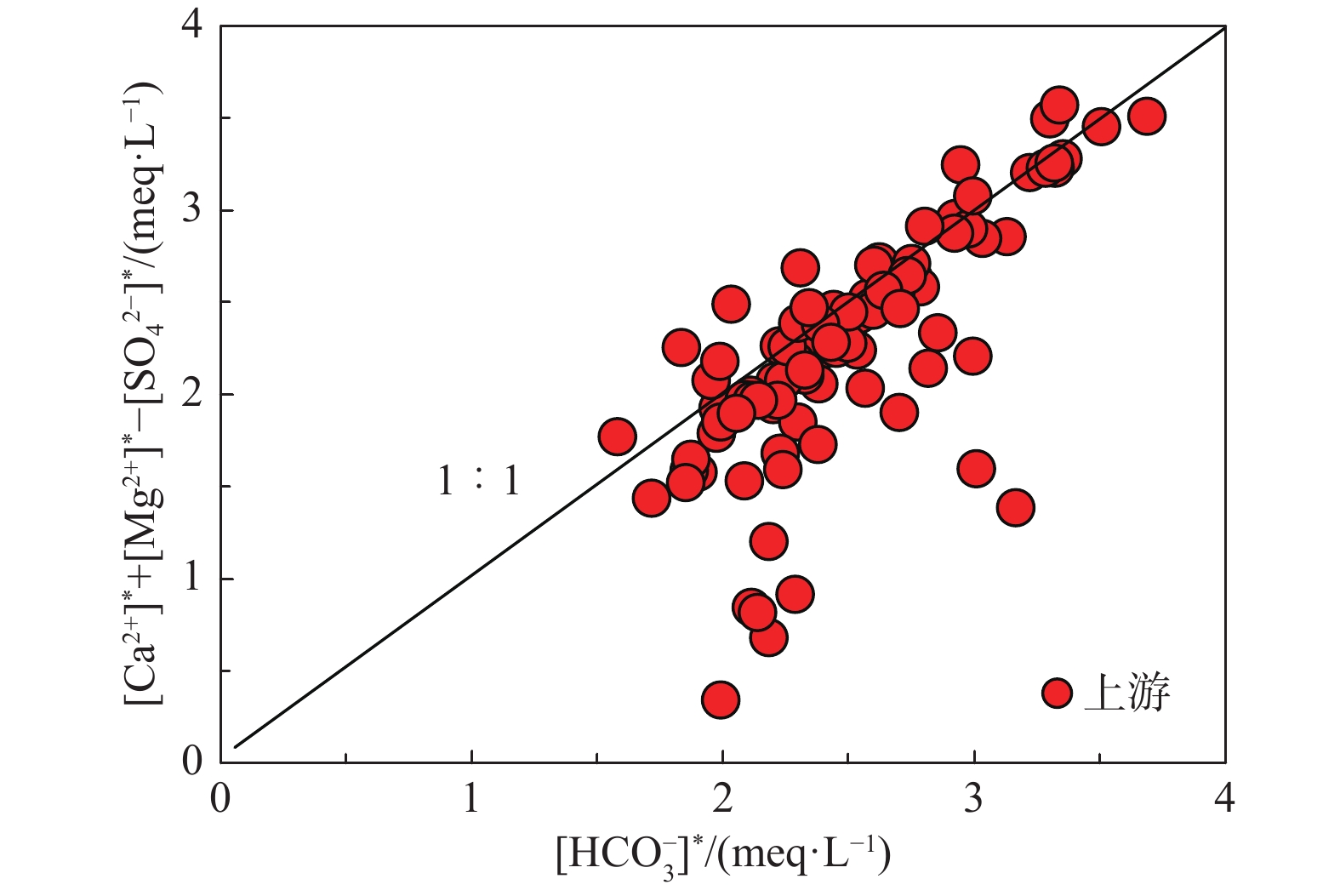
长江流域面积巨大,岩性多变,加之三峡大坝等重大水利工程的影响,干流河水的水化学成因存在较大争议。此外,以往研究中流域矿物风化过程的碳汇通量估算一般基于阳离子来源分析,但该算法通常涉及多种矿物端元的参数选取,结果具有不确定性。本次研究对长江干流水化学的时空演变进行了整体分析,并基于上游河水样品
$\rm{HCO}_3^{-}$ $\rm{NO}_3^{-}$ Abstract:Because river water in the Yangtze River watershed with a huge area is influenced by variable lithologies and large-scale water projects such as the Three Gorges Dam, the hydrochemical origins of the main stream are still controversial. Furthermore, previous estimations on the carbon sink in the watershed caused by mineral weathering are mostly based on the mass-balance calculations of cations, but this method generally involves the selection of parameters of various mineral end-members, which causes the uncertainty of results. In this study, the temporal and spatial evolutions of hydrochemistry of the main stream are determined, and a new method for the determination of CO2 consumption rates during mineral weathering processes are proposed based on the mass-balance calculations of
$\rm{HCO}_3^{-} $ $\rm{NO}_3^{-} $ -

-
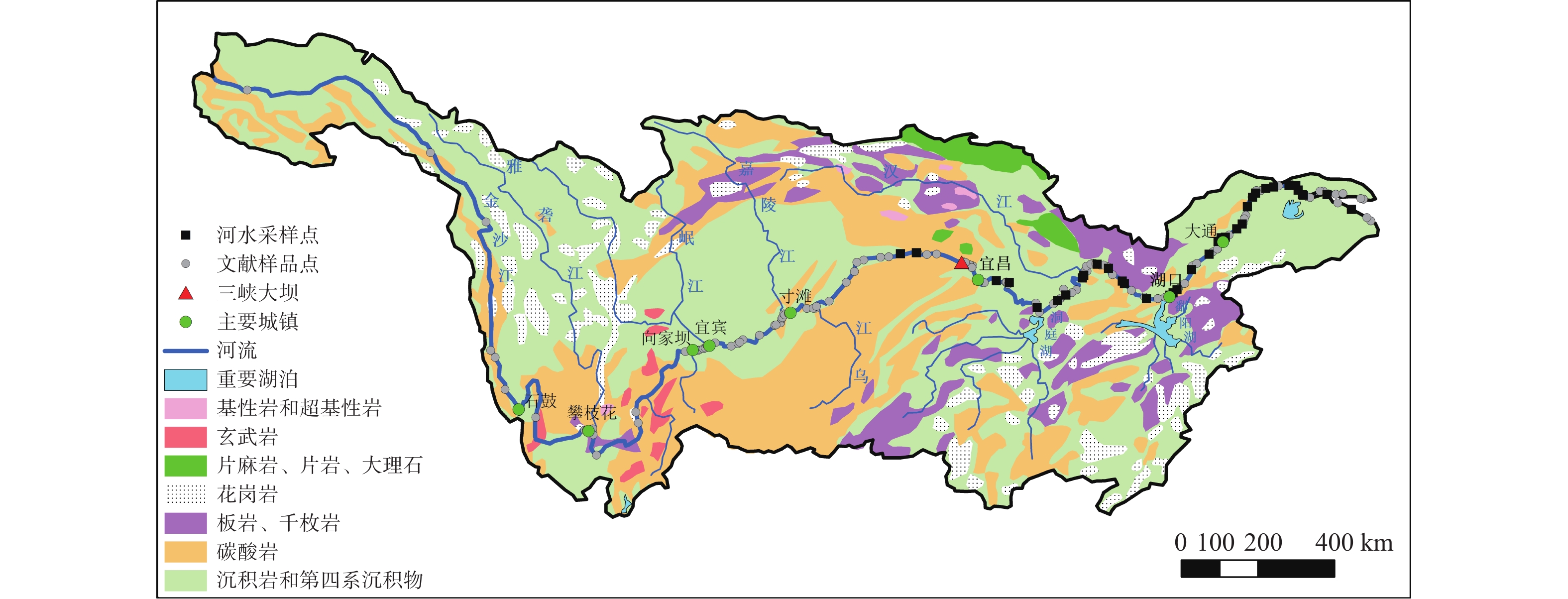
图 1 长江流域水样点及岩石类型分布图(据文献 [9]修改)
Figure 1.
表 1 长江中游与下游河水水化学参数的统计特征值
Table 1. Statistics of hydrochemical parameters of the river water in the middle and lower reaches of the Yangtze River
/(mg·L−1) 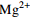
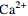
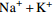
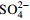

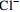
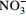
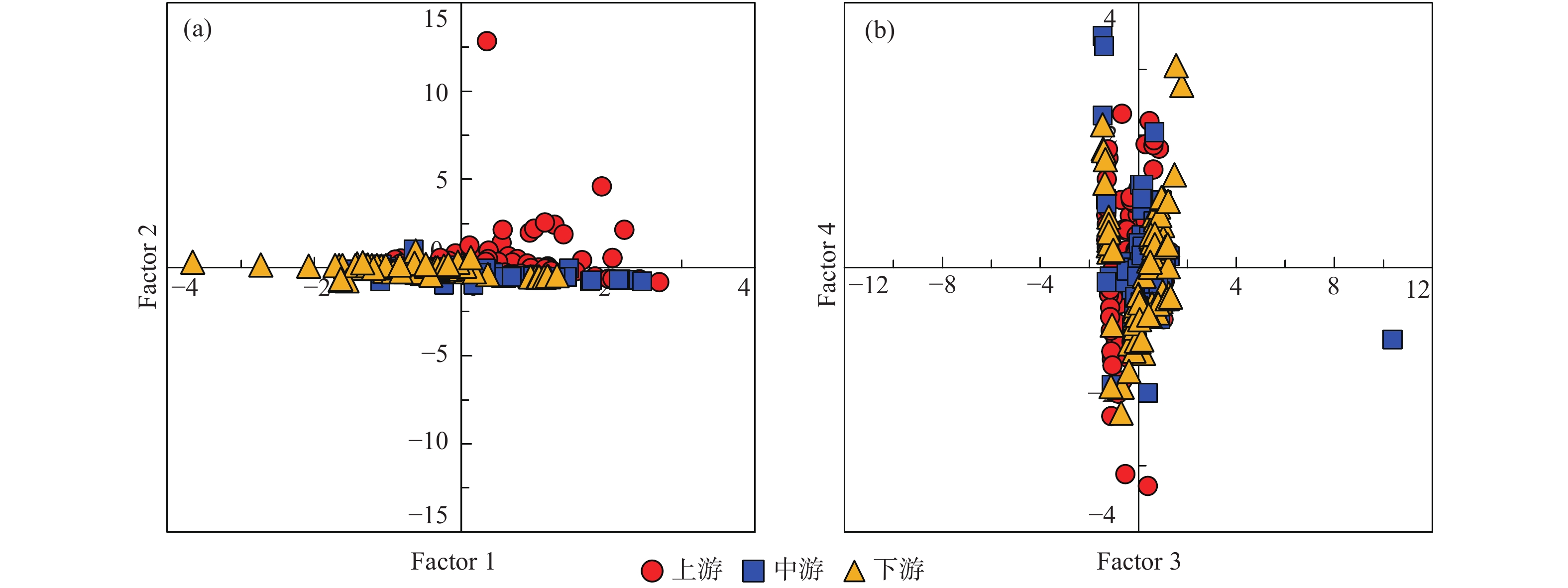
中游 (2007年前) 变化范围 4.46~13.28 30.42~73.40 4.60~16.30 12.30~66.24 85.40~210.50 4.30~17.70 2.54~9.26 平均值 8.84 45.97 10.32 33.51 144.19 10.56 5.27 中游 (2013年后) 变化范围 5.60~9.10 29.30~44.28 10.10~17.60 13.49~43.10 78.97~133.60 4.51~17.30 4.19~47.23 平均值 7.14 36.34 13.36 33.02 113.32 12.45 9.01 下游 (2007年前) 变化范围 2.11~11.08 10.52~53.54 5.00~25.64 10.02~51.40 24.40~165.60 4.20~32.10 0.05~10.60 平均值 7.66 40.19 10.48 30.10 120.94 11.50 6.16 下游 (2013年后) 变化范围 2.72~7.40 18.23~39.70 6.72~35.40 6.79~63.81 46.17~137.50 3.05~34.90 1.88~12.97 平均值 6.33 33.52 14.02 35.68 98.60 15.20 6.91 表 2 旋转后的成分矩阵表
Table 2. Eigenvalue matrix after rotation
变量 成分 因子1 因子2 因子3 因子4 
0.94 0.11 −0.21 −0.11 
0.92 −0.03 0.12 0.18 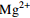
0.83 0.32 −0.10 0.12 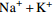
0.14 0.96 −0.10 0.18 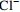
0.13 0.96 −0.04 0.19 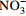
−0.09 −0.09 0.99 0.06 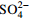
0.10 0.30 0.07 0.94 表 3 长江上游主要支流离子含量
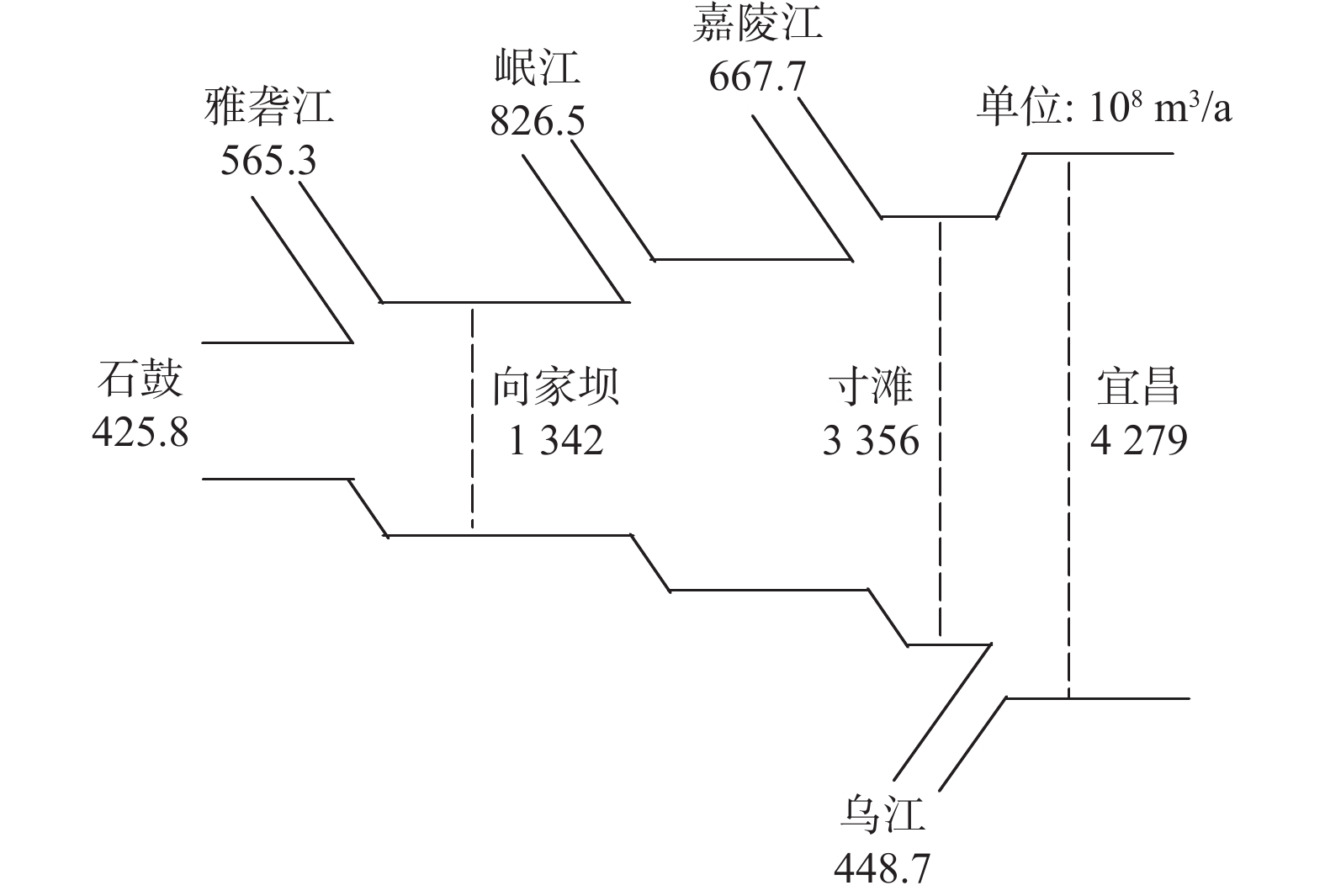
Table 3. Contents of elements carried by tributaries to the upper reaches of the Yangtze River
/(mg·L−1) 支流 Na++K+ Ca2+ Mg2+ Cl− 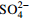

雅砻江 9.62 26.11 8.84 3.20 6.83 103.00 岷江 7.42 32.97 8.45 5.17 20.89 102.86 嘉陵江 9.53 38.48 9.49 6.95 37.62 128.23 乌江 6.91 41.00 9.59 4.03 37.92 127.60 -
[1] GAILLARDET J, DUPRÉ B, LOUVAT P, et al. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers[J]. Chemical Geology,1999,159(1/2/3/4):3 − 30.
[2] 霍俊伊, 于奭, 张清华, 等. 湘西峒河流域水化学特征及无机碳通量计算[J]. 水文地质工程地质,2019,46(4):64 − 72. [HUO Junyi, YU Shi, ZHANG Qinghua, et al. Hydrochemical characteristics and estimation of the dissolved inorganic carbon flux in the Donghe River Basin of western Hunan[J]. Hydrogeology & Engineering Geology,2019,46(4):64 − 72. (in Chinese with English abstract)
[3] 张敏, 平建华, 禹言, 等. 同位素技术解析安阳河与地下水相互作用[J]. 水文地质工程地质,2019,46(6):31 − 39. [ZHANG Min, PING Jianhua, YU Yan, et al. Isotope analyses of the interaction between the Anyang River and groundwater[J]. Hydrogeology & Engineering Geology,2019,46(6):31 − 39. (in Chinese with English abstract)
[4] 吴卫华, 杨杰东, 徐士进. 青藏高原化学风化和对大气CO2的消耗通量[J]. 地质论评,2007,53(4):515 − 528. [WU Weihua, YANG Jiedong, XU Shijin. Chemical weathering and atmospheric CO2 consumption of Qinghai—Xizang plateau[J]. Geological Review,2007,53(4):515 − 528. (in Chinese with English abstract) doi: 10.3321/j.issn:0371-5736.2007.04.011
[5] WU W H, YANG J D, XU S J, et al. Geochemistry of the headwaters of the Yangtze River, Tongtian He and Jinsha Jiang: silicate weathering and CO2 consumption[J]. Applied Geochemistry,2008,23(12):3712 − 3727. doi: 10.1016/j.apgeochem.2008.09.005
[6] 陶正华, 赵志琦, 张东, 等. 西南三江(金沙江、澜沧江和怒江)流域化学风化过程[J]. 生态学杂志,2015,34(8):2297 − 2308. [TAO Zhenghua, ZHAO Zhiqi, ZHANG Dong, et al. Chemical weathering in the Three Rivers (Jingshajiang, Lancangjiang, and Nujiang) watershed, southwest China[J]. Chinese Journal of Ecology,2015,34(8):2297 − 2308. (in Chinese with English abstract)
[7] 张连凯, 覃小群, 刘朋雨, 等. 硫酸参与的长江流域岩石化学风化与大气CO2消耗[J]. 地质学报,2016,90(8):1933 − 1944. [ZHANG Liankai, QIN Xiaoqun, LIU Pengyu, et al. Chemical denudation rate and atmospheric CO2 consumption by H2CO3 and H2SO4 in the Yangtze River Catchment[J]. Acta Geologica Sinica,2016,90(8):1933 − 1944. (in Chinese with English abstract) doi: 10.3969/j.issn.0001-5717.2016.08.021
[8] 汪齐连, 刘丛强, 赵志琦, 等. 长江流域河水和悬浮物的锂同位素地球化学研究[J]. 地球科学进展,2008,23(9):952 − 958. [WANG Qilian, LIU Congqiang, ZHAO Zhiqi, et al. Lithium isotopic composition of the dissolved and suspended loads of the Yangtze River, China[J]. Advances in Earth Science,2008,23(9):952 − 958. (in Chinese with English abstract) doi: 10.3321/j.issn:1001-8166.2008.09.006
[9] LUO C, ZHENG H B, TADA R, et al. Tracing Sr isotopic composition in space and time across the Yangtze River basin[J]. Chemical Geology,2014,388:59 − 70. doi: 10.1016/j.chemgeo.2014.09.007
[10] WANG Z L, ZHANG J, LIU C Q. Strontium isotopic compositions of dissolved and suspended loads from the main channel of the Yangtze River[J]. Chemosphere,2007,69(7):1081 − 1088. doi: 10.1016/j.chemosphere.2007.04.031
[11] CHEN J S, WANG F Y, XIA X H, et al. Major element chemistry of the Changjiang (Yangtze River)[J]. Chemical Geology,2002,187(3/4):231 − 255.
[12] 简慧敏, 姚庆祯, 张经, 等. 长江流域常量元素的分布特征[J]. 长江流域资源与环境,2010,19(1):93 − 97. [JIAN Huimin, YAO Qingzhen, ZHANG Jing, et al. Distribution characteristics of major element in the Changjiang river[J]. Resources and Environment in the Yangtze Basin,2010,19(1):93 − 97. (in Chinese with English abstract)
[13] DING T P, GAO J F, TIAN S H, et al. Chemical and isotopic characteristics of the water and suspended particulate materials in the Yangtze River and their geological and environmental implications[J]. Acta Geologica Sinica - English Edition,2014,88(1):276 − 360. doi: 10.1111/1755-6724.12197
[14] 李小倩, 刘运德, 周爱国, 等. 长江干流丰水期河水硫酸盐同位素组成特征及其来源解析[J]. 地球科学,2014,39(11):1647 − 1654. [LI Xiaoqian, LIU Yunde, ZHOU Aiguo, et al. Sulfur and oxygen isotope compositions of dissolved sulfate in the Yangtze River during high water period and its sulfate source tracing[J]. Earth Science,2014,39(11):1647 − 1654. (in Chinese with English abstract)
[15] DELANY J M, LUNDEEN S R. The LLNL thermochemical database[R]. Lawrence Livermore National Laboratory Report (UCRL-21658), 1990.
[16] PARKHURST D L, APPLO C A J. User’s guide to PHREEQC: A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations[R]. 2nd ed. Reston: US Geological Survey, 1999.
[17] 陈静生, 王飞越, 夏星辉. 长江水质地球化学[J]. 地学前缘,2006,13(1):74 − 85. [CHEN Jinsheng, WANG Feiyue, XIA Xinghui. Geochemistry of water quality of the Yangtze River basin[J]. Earth Science Frontiers,2006,13(1):74 − 85. (in Chinese with English abstract) doi: 10.3321/j.issn:1005-2321.2006.01.010
[18] 李丹, 邓兵, 张国森, 等. 近年来长江口水体主离子的变化特征及影响因素分析[J]. 华东师范大学学报(自然科学版),2010(2):34 − 42. [LI Dan, DENG Bing, ZHANG Guosen, et al. Change characteristics of major ions and their influence factors in Yangtze Estuary in recent years[J]. Journal of East China Normal University (Natural Science),2010(2):34 − 42. (in Chinese with English abstract)
[19] WU Q X, HAN G L. Sulfur isotope and chemical composition of the rainwater at the Three Gorges Reservoir[J]. Atmospheric Research,2015,155:130 − 140. doi: 10.1016/j.atmosres.2014.11.020
[20] CHETELAT B, LIU C Q, ZHAO Z Q, et al. Geochemistry of the dissolved load of the Changjiang Basin rivers: Anthropogenic impacts and chemical weathering[J]. Geochimica et Cosmochimica Acta,2008,72(17):4254 − 4277. doi: 10.1016/j.gca.2008.06.013
[21] WANG X D, YANG S Y, RAN X B, et al. Response of the Changjiang (Yangtze River) water chemistry to the impoundment of Three Gorges Dam during 2010-2011[J]. Chemical Geology,2018,487:1 − 11. doi: 10.1016/j.chemgeo.2018.04.006
[22] MAVROMATIS V, GAUTIER Q, BOSC O, et al. Kinetics of Mg partition and Mg stable isotope fractionation during its incorporation in calcite[J]. Geochimica et Cosmochimica Acta,2013,114:188 − 203. doi: 10.1016/j.gca.2013.03.024
[23] GIBBS R J. Mechanisms controlling world water chemistry[J]. Science,1970,170(3962):1088 − 1090. doi: 10.1126/science.170.3962.1088
[24] 杨楠, 苏春利, 曾邯斌, 等. 基于水化学和氢氧同位素的兴隆县地下水演化过程研究[J]. 水文地质工程地质,2020,47(6):154 − 162. [YANG Nan, SU Chunli, ZENG Hanbin, et al. Evolutional processes of groundwater in Xinglong County based on hydrochemistry and hydrogen and oxygen isotopes[J]. Hydrogeology & Engineering Geology,2020,47(6):154 − 162. (in Chinese with English abstract)
[25] LI Z J, LI Z X, SONG L L, et al. Precipitation chemistry in the Source Region of the Yangtze River[J]. Atmospheric Research,2020,245:105073. doi: 10.1016/j.atmosres.2020.105073
[26] JIANG L G, YAO Z J, LIU Z F, et al. Hydrochemistry and its controlling factors of rivers in the source region of the Yangtze River on the Xizang Plateau[J]. Journal of Geochemical Exploration,2015,155:76 − 83. doi: 10.1016/j.gexplo.2015.04.009
[27] 夏学齐, 杨忠芳, 王亚平, 等. 长江水系河水主要离子化学特征[J]. 地学前缘,2008,15(5):194 − 202. [XIA Xueqi, YANG Zhongfang, WANG Yaping, et al. Major ion chemistry in the Yangtze River[J]. Earth Science Frontiers,2008,15(5):194 − 202. (in Chinese with English abstract) doi: 10.3321/j.issn:1005-2321.2008.05.020
[28] 吴文涛, 冉祥滨, 李景喜, 等. 长江水体常量和微量元素的来源、分布与向海输送[J]. 环境科学,2019,40(11):4900 − 4913. [WU Wentao, RAN Xiangbin, LI Jingxi, et al. Sources, distribution, and fluxes of major and trace elements in the Yangtze River[J]. Environmental Science,2019,40(11):4900 − 4913. (in Chinese with English abstract)
[29] 中华人民共和国水利部. 中国河流泥沙公报(2019)[M]. 北京: 中国水利水电出版社, 2019.
Ministry of Water Resources of the People's Republic of China. China river sediment bulletin (2019)[M]. Beijing: China Water Resources and Hydropower Press, 2019. (in Chinese)
[30] 许越先, 谢明, 张永忠. 长江上游离子径流量的估算及时空变化特征[J]. 地理研究,1991,10(4):19 − 28. [XU Yuexian, XIE Ming, ZHANG Yongzhong. Calculation of amount of the chemical flow at the upper reaches of Changjiang River and analysis of its temporal and spatial variation[J]. Geographical Research,1991,10(4):19 − 28. (in Chinese with English abstract)
-







 下载:
下载: