Ability of Modified Slag to Treat Reactive Brilliant Blue KN-R Dye Wastewater
-
摘要:
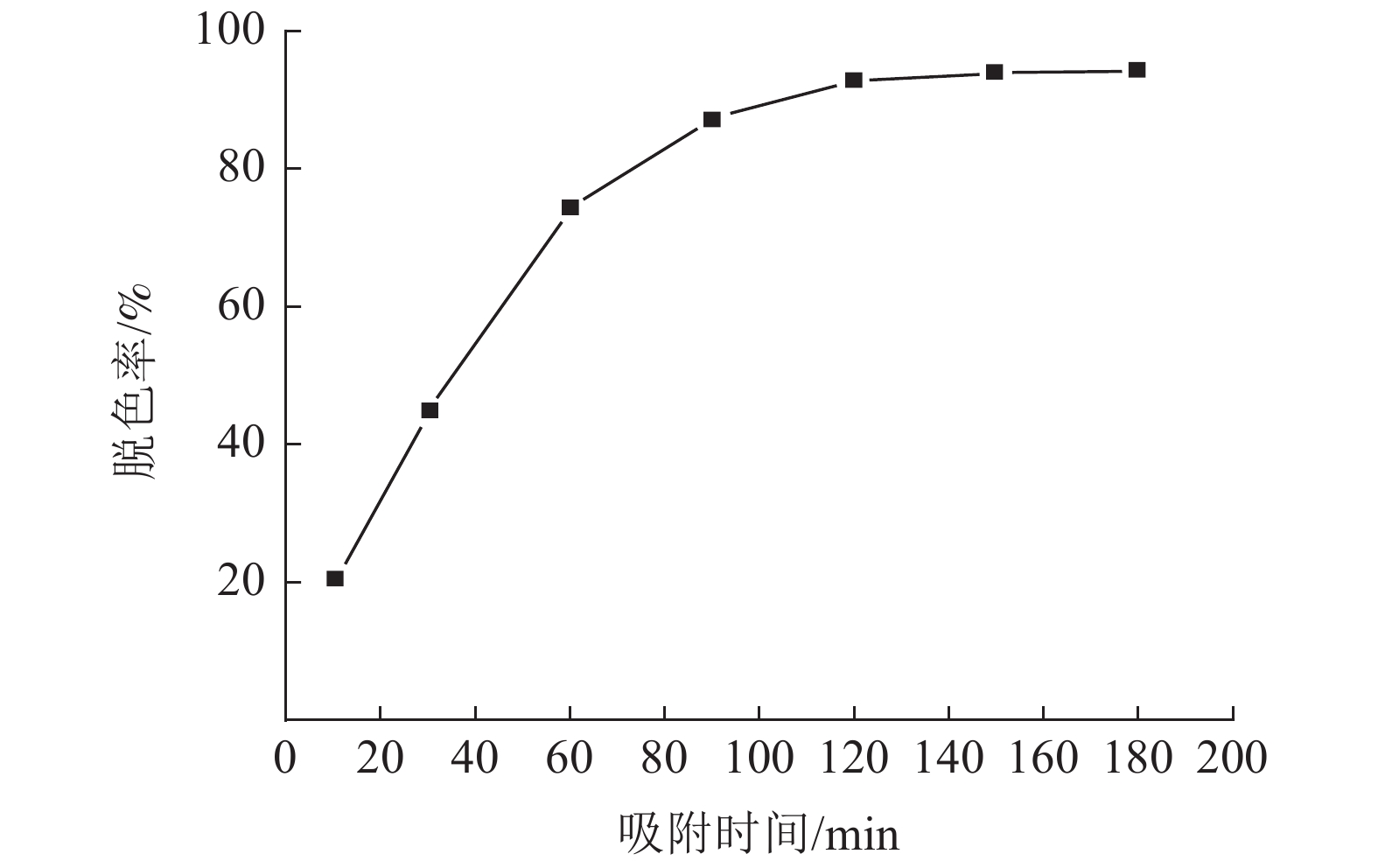
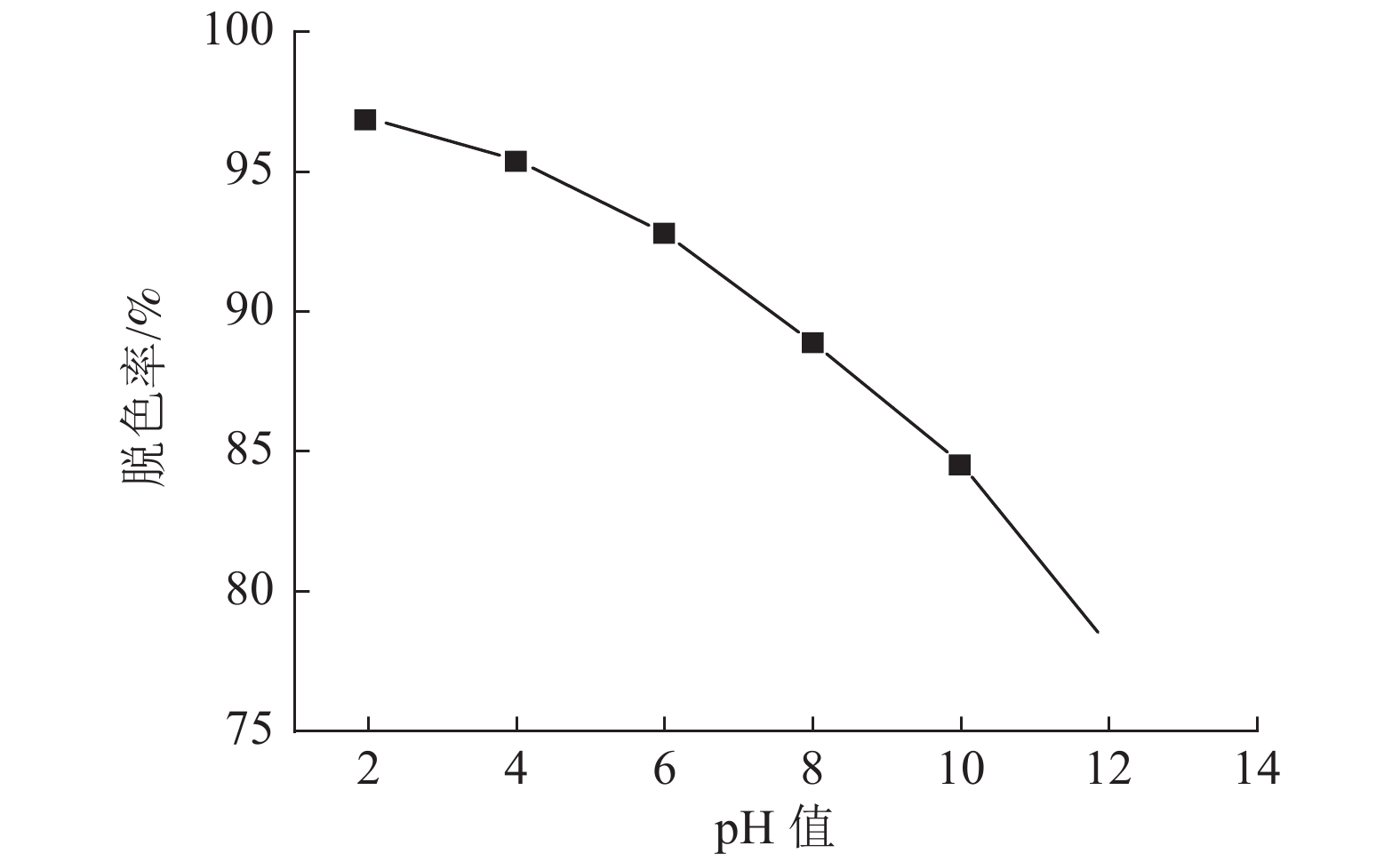
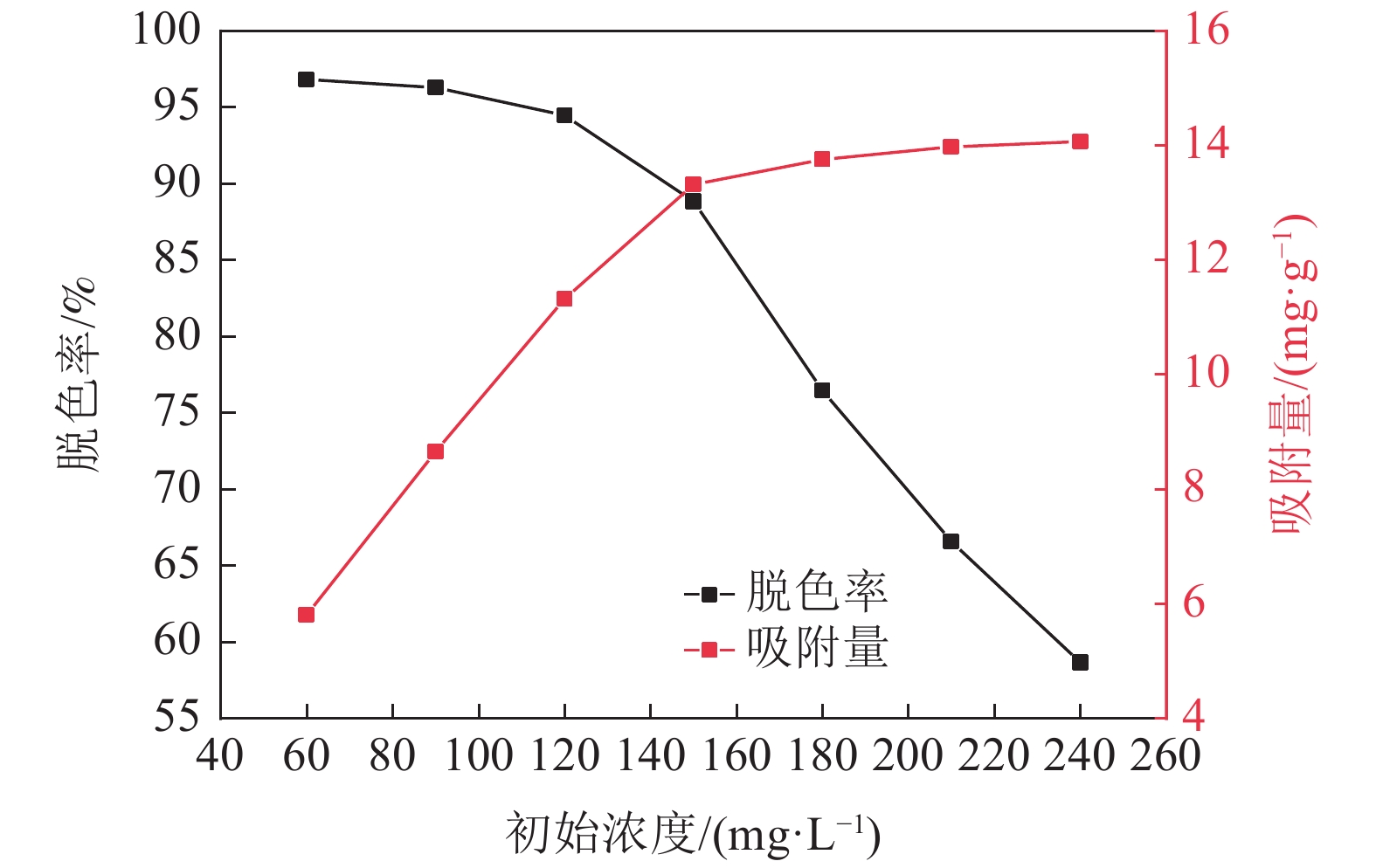
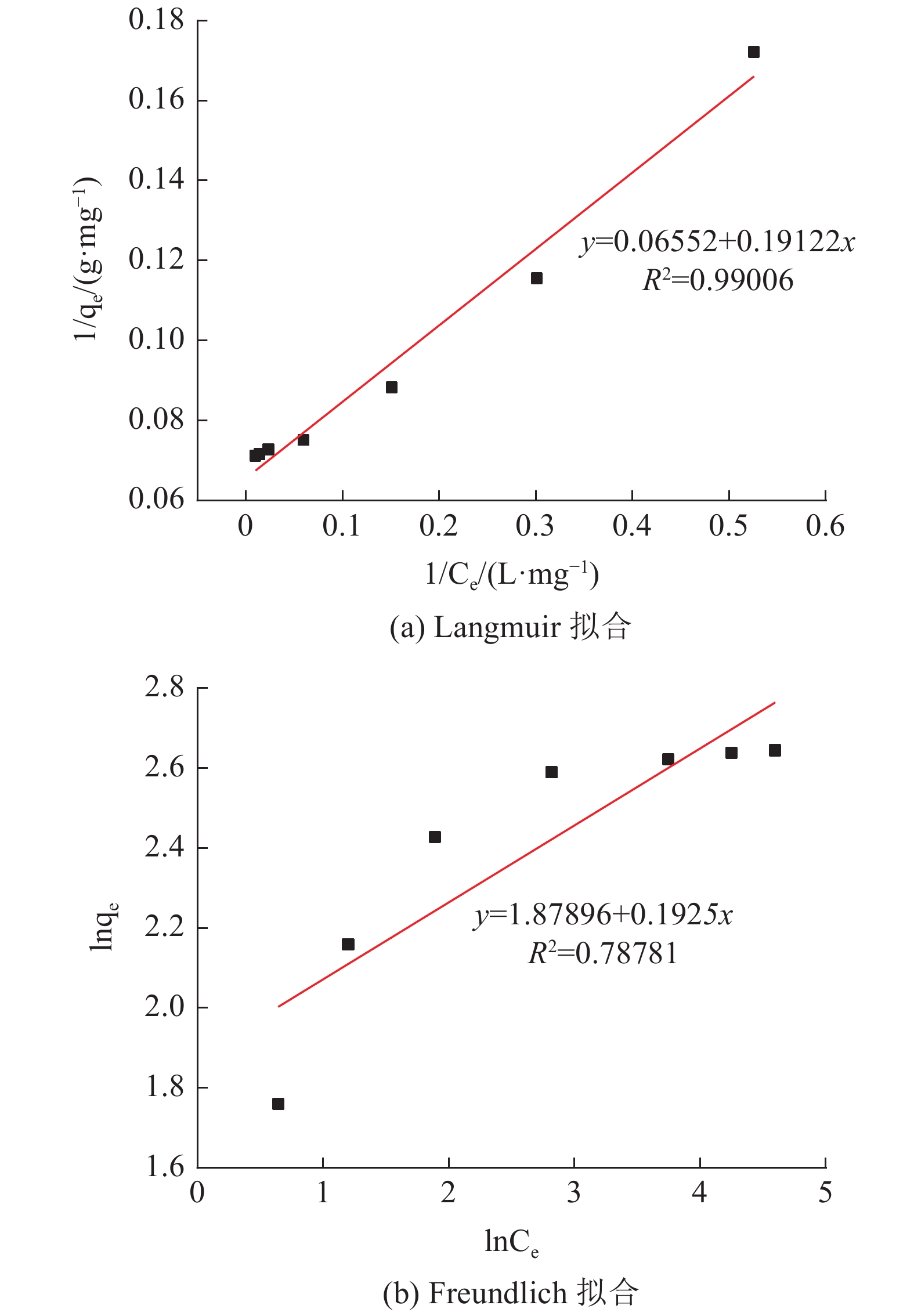
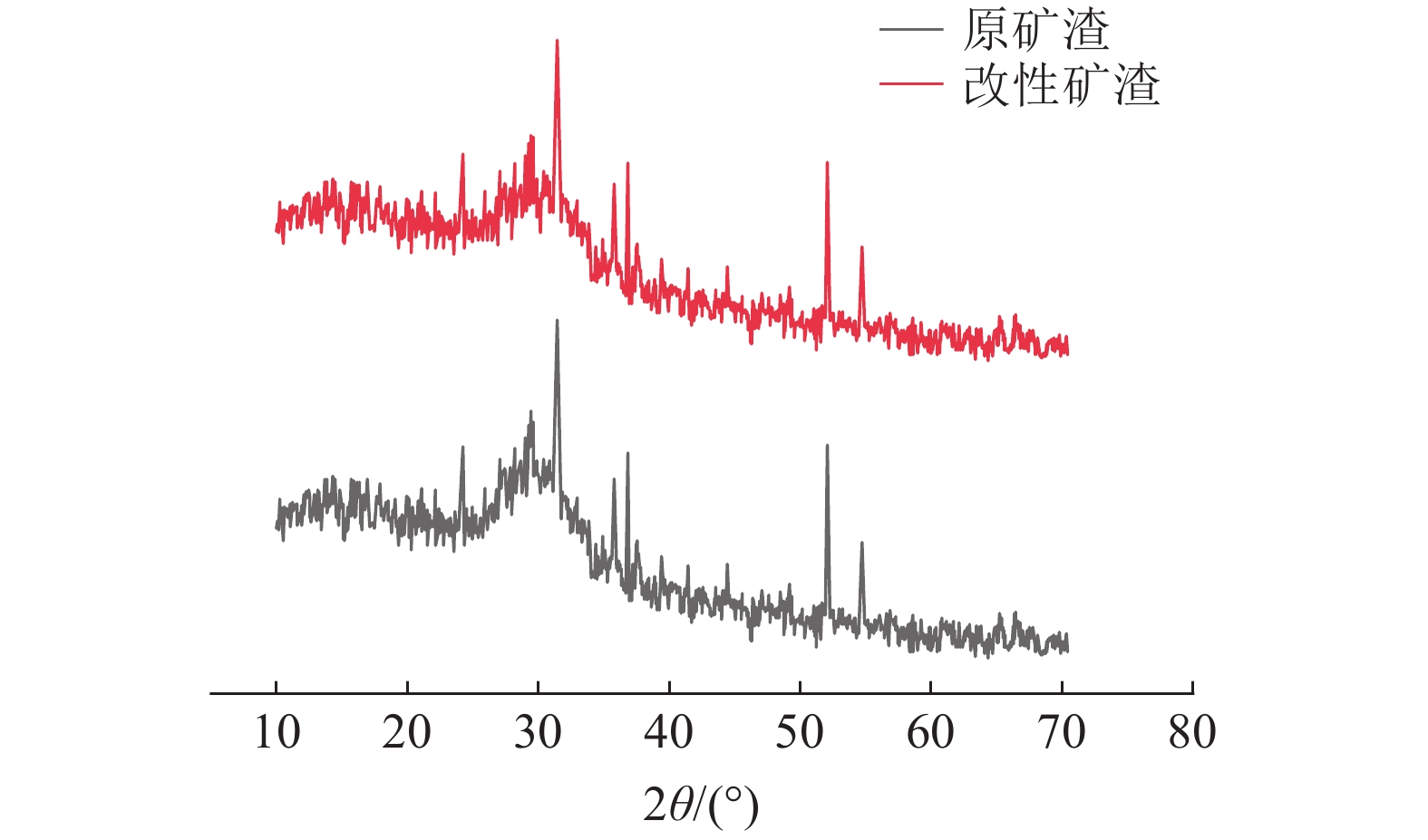
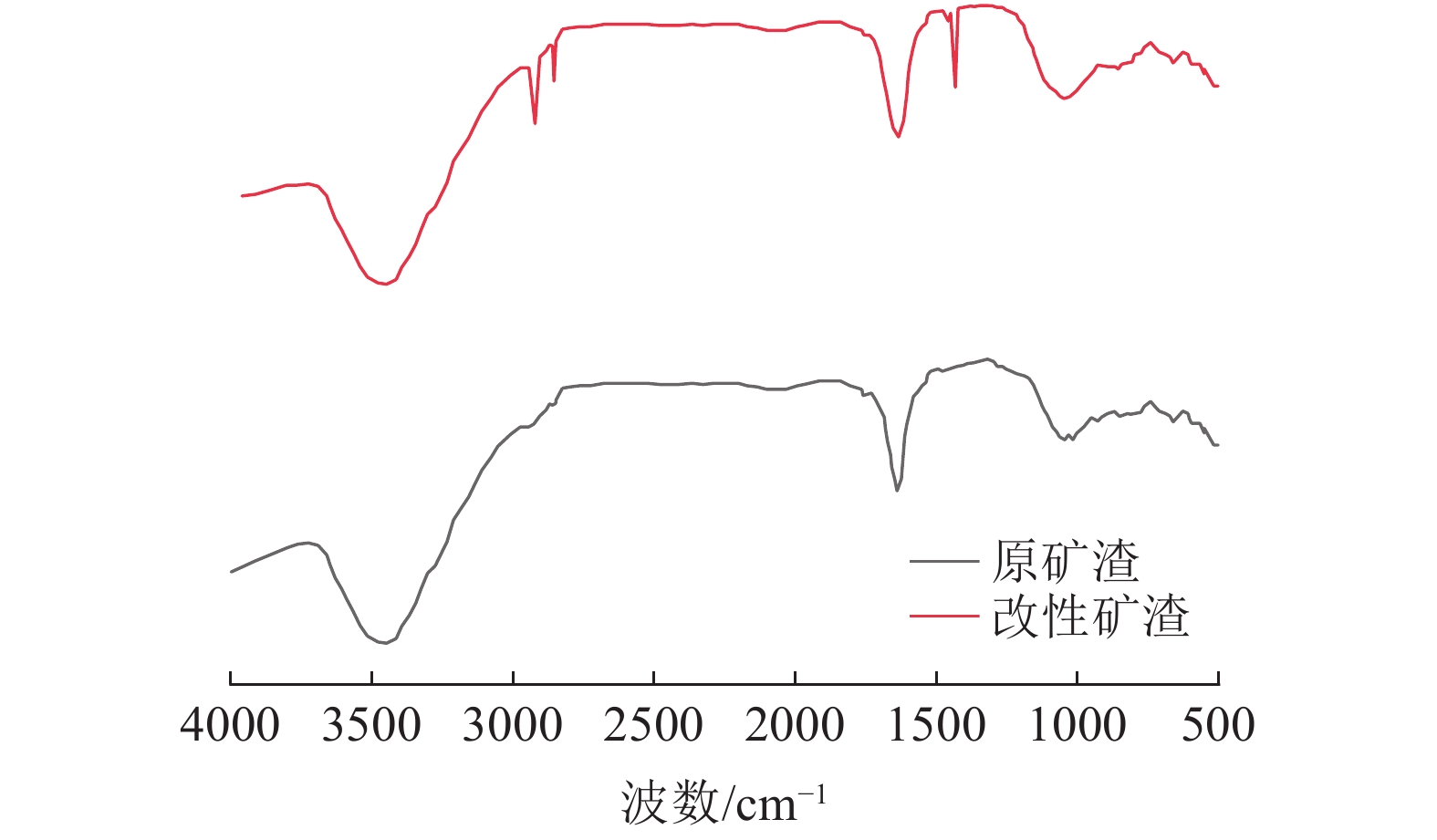
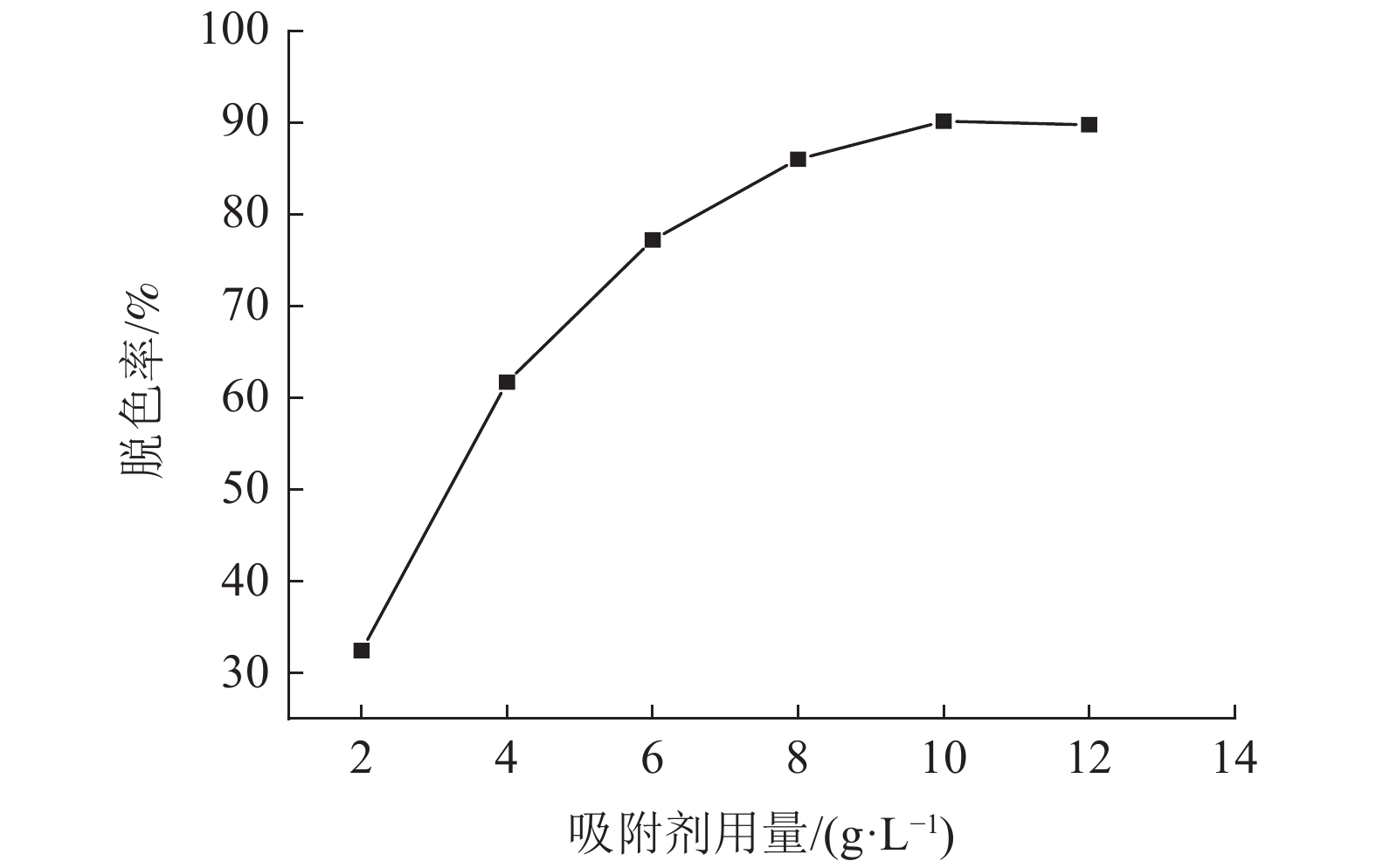
采用十二烷基三甲基氯化铵对高炉矿渣进行改性,制备一种低成本、环境友好型的吸附剂来处理染料废水,研究分析了吸附剂剂量、吸附时间、染料初始浓度和pH值因素对改性矿渣吸附活性艳蓝KN-R能力的影响以及改性矿渣表征的变化规律。结果表明:当改性矿渣用量为10 g/L,染料初始浓度为60 mg/L,pH值为2,以及吸附120 min时,改性矿渣对污水的处理后可达到较大脱色率96%,而在初始浓度为150 mg/L时为较优溶度,该浓度下脱色率与吸附量均较高。通过XRD和FTIR实验对矿渣与改性矿渣的表征特点进行对比分析,分析表明十二烷基三甲基氯化铵可以有效地对矿渣进行改性,改性后的矿渣吸附能力得到了显著提升。
Abstract:The blast furnace slag was modified with DTAC to prepare a low-cost and environment-friendly adsorbent for the treatment of dye wastewater. The study analyzes theadsorbent dose, adsorption time, initial dye concentration and pH value on the adsorption capacity of KN-R reactive brilliant blue from the modified slag , and the variation rule of characterization of the modified slag was also analyzed. The results show that when the amount of modified slag is 10 g/L, the initial concentration of dye is 60 mg/L, the pH value is 2, and the adsorption time is 120 min, the maximum decolorization rate of the modified slag can reach 96%, and the initial concentration of 150 mg/L is the optimal solubility, the decolorization rate and adsorption capacity are both high at this concentration. Through XRD and FTIR tests, the characterization characteristics of slag and modified slag were compared and analyzed. It was found that DTAC could better modify slag, and the adsorption capacity of modified slag was significantly improved.
-

-
[1] Pang X, Sellaoui L, Franco D, et al. Preparation and characterization of a novel mountain soursop seeds powder adsorbent and its application for the removal of crystal violet and methylene blue from aqueous solutions[J]. Chemical Engineering Journal, 2019, 391:123617.
[2] 张中领, 孙晓玲. 染料废水处理技术现状与发展[J]. 化工设计通讯, 2017, 43(3):205. doi: 10.3969/j.issn.1003-6490.2017.03.165
ZHANG Z L, SUN X L. Status and development of dye wastewater treatment technology[J]. Chemical Design Communication, 2017, 43(3):205. doi: 10.3969/j.issn.1003-6490.2017.03.165
[3] 秦彬, 谷晋川, 殷萍, 等. 染料废水处理技术研究进展[J]. 化工环保, 2021, 41(1):9-18. doi: 10.3969/j.issn.1006-1878.2021.01.002
QIN B, GU J C, YIN P, et al. Research progress of dyestuff waste water treatment technology[J]. Chemical Environmental Protection, 2021, 41(1):9-18. doi: 10.3969/j.issn.1006-1878.2021.01.002
[4] Yagub M T, Sen T K, Afroze S, et al. Dye and its removal from aqueous solution by adsorption: a review – science direct[J]. Advances in Colloid and Interface Science, 2014, 209(1):172-184.
[5] 陈伟, 贾献峰. 膨胀石墨对刚果红废水吸附及动力学研究[J]. 南开大学学报(自然科学版), 2021, 54(2):85-91.
CHEN W, JIA X F. Study on the adsorption and kinetics of Congo red wastewater by dye waste expanded graphite[J]. Journal of Nankai University(Natural Science Edition), 2021, 54(2):85-91.
[6] Sahar Rastegar Koohi, Somaiyeh Allahyari, Davood Kahforooshan, et al. Natural minerals as support of silicotungstic acid for photocatalytic degradation of methylene blue in wastewater[J]. Springer US, 2019, 29(2).
[7] 严岩, 苏仕军, 廖兵, 等. 针铁矿渣对刚果红模拟染料废水的吸附特性[J]. 湿法冶金, 2018, 37(1):45-49+69.
YAN Y, SU S J, LIAO B, et al. Adsorption characteristics of dye waste goethite slag on Congo red simulated dye wastewater[J]. Hydrometallurgy, 2018, 37(1):45-49+69.
[8] 邓文静. 木屑季铵盐型螯合吸附剂的制备及其对废水中铀(Ⅵ)吸附的试验研究[D]. 衡阳: 南华大学, 2015.
DENG W J. Preparation of dye waste wood chips quaternary ammonium salt type chelating adsorbent and its experimental study on the adsorption of uranium (Ⅵ) in wastewater [D]. Hengyang: University of South China, 2015.
[9] Liu Kai, Zhang Siyu, Hu Xiyue, et al. Understanding the adsorption of PFOA on MIL-101(Cr)-based anionic-exchange metal-organic frameworks: comparing DFT calculations with aqueous sorption experiments[J]. Environmental Science & Technology, 2015, 49(14).
[10] 贺君, 邢丽飞, 王彩, 等. 改性沸石去除橙黄G染料废水试验研究[J]. 非金属矿, 2018, 41(1):80-83. doi: 10.3969/j.issn.1000-8098.2018.01.024
HE J, XING L F, WANG C, et al. Experimental study on the removal of orange-yellow G dye wastewater by dye waste modified zeolite[J]. Nonmetallic Minerals, 2018, 41(1):80-83. doi: 10.3969/j.issn.1000-8098.2018.01.024
[11] Mansur H S, RL Oréfice, Mansur A. Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy[J]. Polymer, 2004, 45(21):7193-7202. doi: 10.1016/j.polymer.2004.08.036
-



 下载:
下载: