Study on Dynamic Adsorption of Copper in Water by Thermally Modified Fly Ash
-
摘要:
以热改性粉煤灰作为吸附剂,采用固定床吸附装置,探究了床层高度、流量、初始浓度等因素对Cu2+动态吸附曲线的影响。在此基础上进行了动态吸附模型的研究,分别研究了Thomas、Yoon-Nelson和Adams-Bohart三种吸附模型。同时也探讨了双组分污染物体系中MFA对Cu2+的动态吸附效果。结果表明,Cu2+的穿透时间随初始离子浓度和流量的增加而缩短,随床层高度的增加而延长。MFA吸附Cu2+的动态行为符合Thomas和Yoon-Nelson模型。降低床层高度、增加初始浓度和流量可以提高Cu2+的吸附速率。根据MFA吸附Cu2+前后的表征,吸附主要机理包括含氧官能团与Cu2+的络合和Na+等阳离子与Cu2+的离子交换。在双组分污染物体系中,溶液中的Zn2+、Pb2+对MFA的Cu2+吸附均产生抑制作用,其影响大小为Pb2+>Zn2+。
Abstract:The thermally modified fly ash was applied to adsorb Cu2+ in the fixed-bed column. The effects of bed height, flow rate and initial concentration on the dynamic adsorption curve of Cu2+ were investigated. On such a basis, the adsorption behavior was fitted by dynamic adsorption models. In addition, the dynamic adsorption effect of MFA on Cu2+ in the binary system was investigated. The results showed that the breakthrough time increased with the decrease of initial concentration and flow rate and the increase of bed height. The dynamic adsorption data was well fitted by the Thomas and Yoon-Nelson models. Decreasing the bed height, increasing the initial concentration and the flow rate were conducive to improve the adsorption rate. According to the characterization of MFA before and after the adsorption, the mechanisms of Cu2+ adsorption mainly include the complexation with oxygen-containing functional groups and the cation exchange. The presence of Zn2+ and Pb2+ had the inhibition effect on the adsorption performance of Cu2+,and the influence followed the order of Pb2+>Zn2+.
-
Key words:
- Thermally modified fly ash /
- Dynamic adsorption /
- Cu2+ /
- Adsorption model
-

-
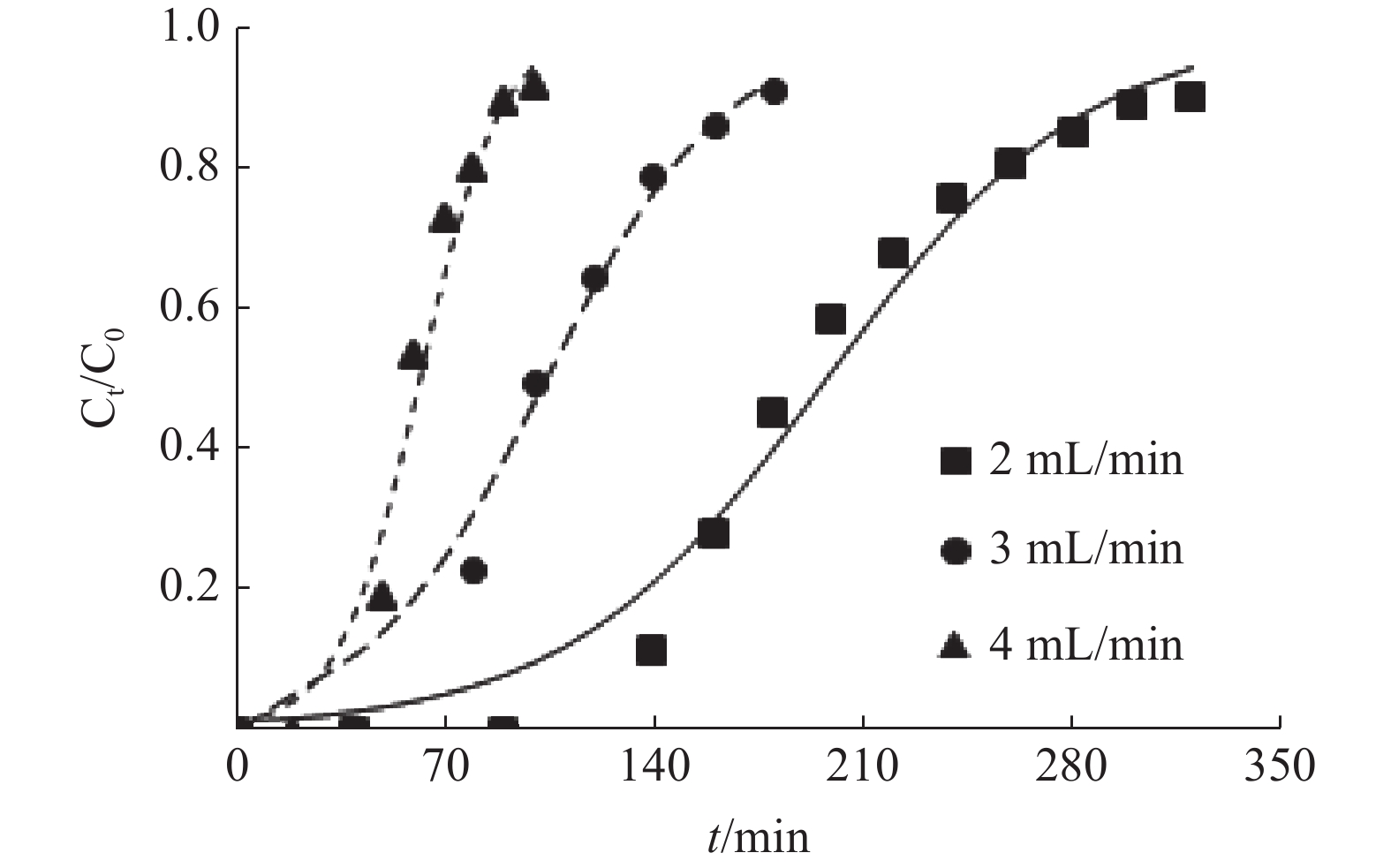
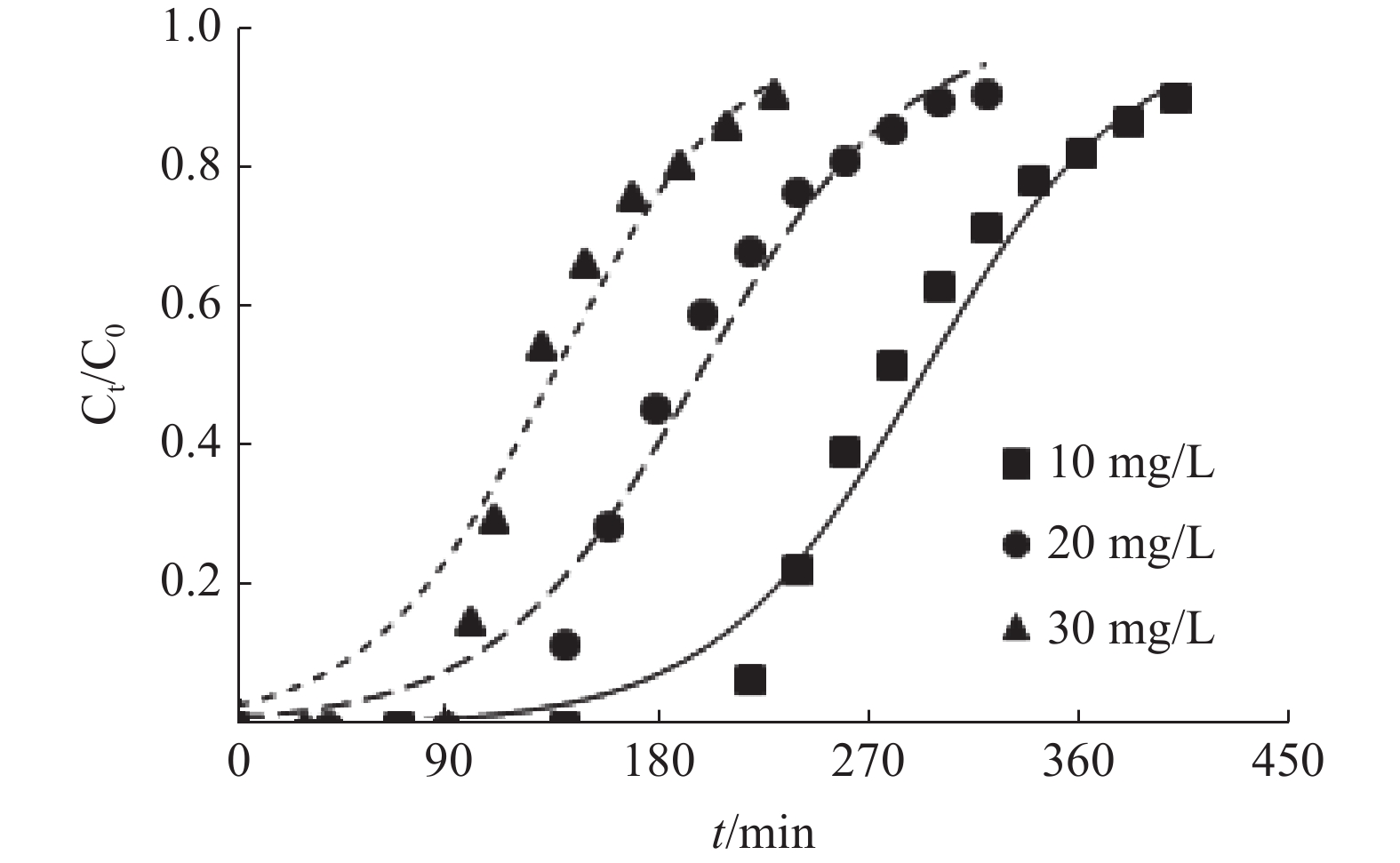
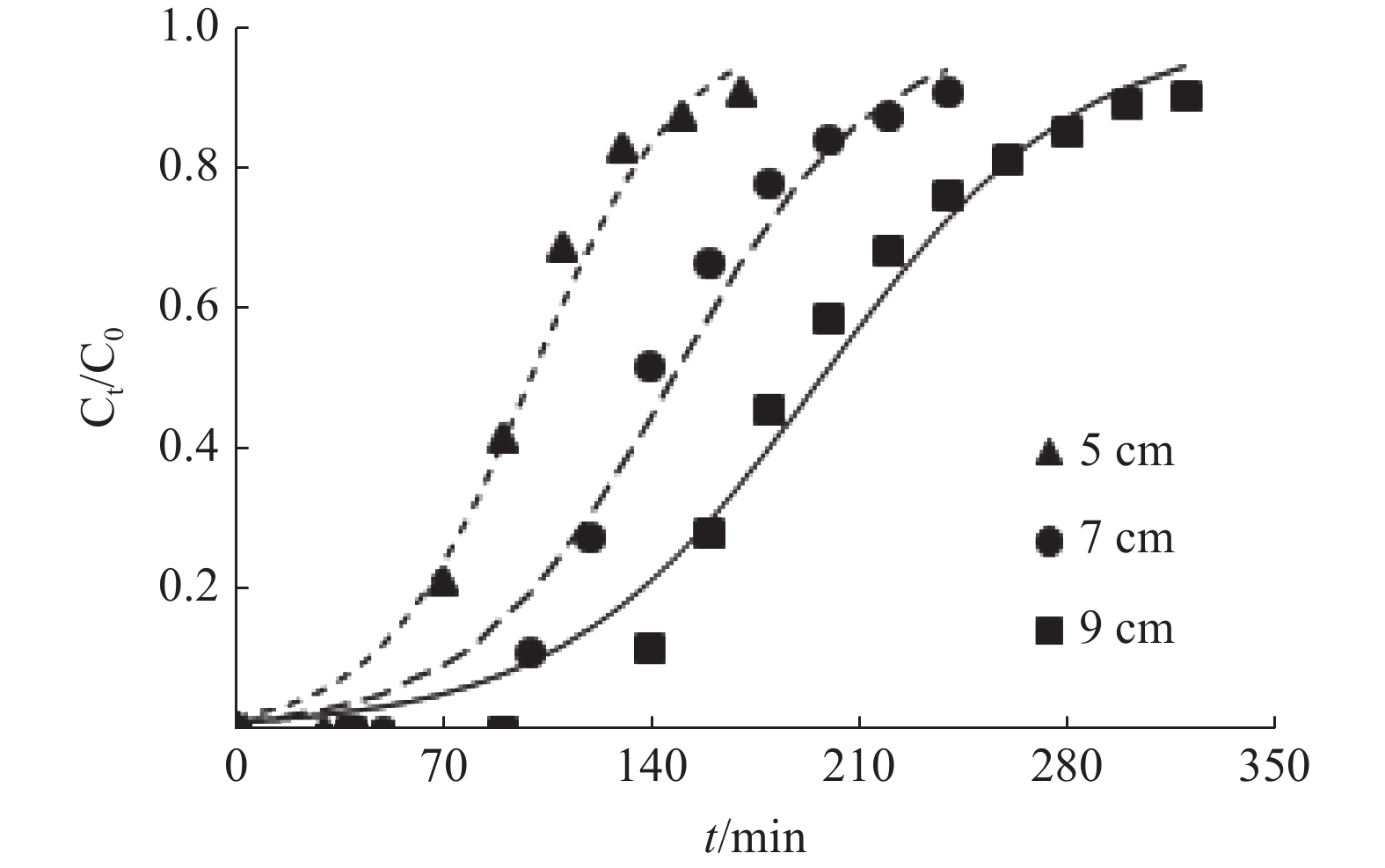
表 1 MFA对Cu2+的动态吸附参数
Table 1. Dynamic adsorption parameters of Cu2+ on MFA
Q
/(mL·min-1)C0
/(mg·L-1)H
/mqe,exp
/(mg·g-1)tb
/mint0.5
/minte
/minη
/%2 20 5 1.84 33 97 170 61.69 2 20 7 1.91 100 138 240 63.47 2 20 9 2.04 133 184 310 67.45 3 20 9 1.71 35 100 180 64.92 4 20 9 1.27 26 59 102 63.81 2 10 9 1.42 224 278 410 71.06 2 30 9 2.14 67 125 230 62.03 表 2 Thomas模型的拟合参数
Table 2. Parameters of Thomas model under different conditions
Q/
(mL·min-1)c0/
(mg·L-1)H/
mThomas模型参数 kT/( mL·(min·mg)-1) qe/(mg·g-1) R2 2 20 5 1.97 1.75 0.922 2 20 7 1.47 1.85 0.935 2 20 9 1.15 1.93 0.947 3 20 9 1.66 1.51 0.973 4 20 9 3.69 1.18 0.935 2 10 9 2.24 1.43 0.932 2 30 9 0.86 1.99 0.920 表 3 Yoon-Nelson模型的拟合参数
Table 3. Parameters of Yoon-Nelson model under different conditions
Q/
(mL·min-1)c0/
(mg·L-1)H/
mYoon-Nelson模型参数 kYN/(min-1) τ/min R2 2 20 5 0.039 100 0.922 2 20 7 0.029 147 0.935 2 20 9 0.023 198 0.947 3 20 9 0.033 104 0.973 4 20 9 0.074 62 0.935 2 10 9 0.022 292 0.932 2 30 9 0.026 125 0.920 表 4 Adams-Bohart模型的拟合参数
Table 4. Parameters of Adams-Bohart model under different conditions
Q/
(mL·min-1)c0/
(mg·L-1)H/
mAdams-Bohart模型参数 kAB/
(10-3 L·mg-1 min-1)N0/(mg·L-1) R2 2 20 5 0.79 1238.22 0.726 2 20 7 0.63 1242.01 0.746 2 20 9 0.48 1274.54 0.755 3 20 9 0.64 1155.98 0.826 4 20 9 1.35 874.59 0.724 2 10 9 0.82 856.67 0.774 2 30 9 0.33 1441.67 0.702 表 5 双组分体系中Cu2+的动态吸附模型拟合参数
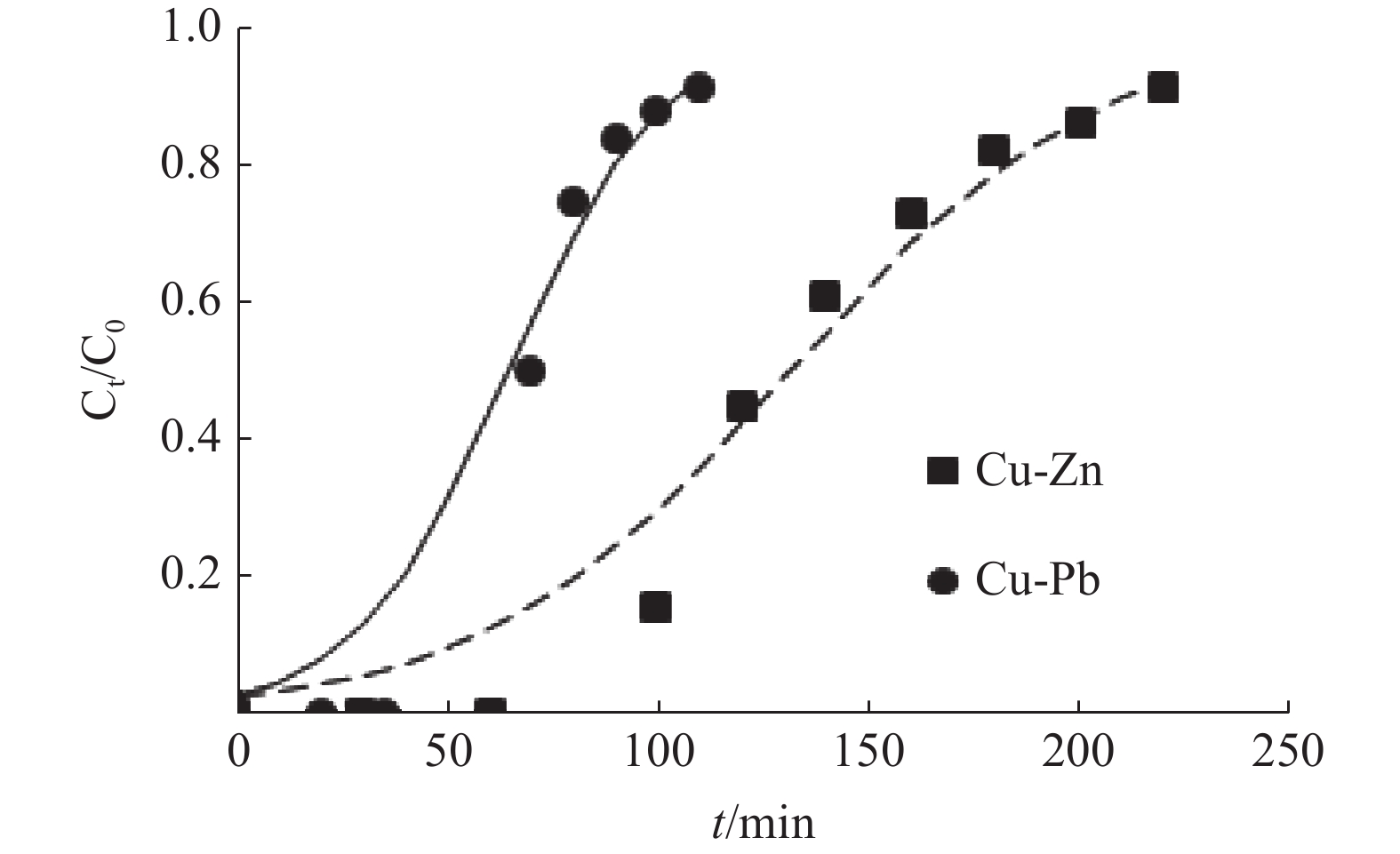
Table 5. Parameters of different models in the binary system
二元体系 Thomas模型 Yoon-Nelson模型 kT/(mL·(min·mg)-1) qe/(mg·g-1) R2 kYN/(min-1) τ/min R2 Cu-Zn 1.36 1.29 0.936 0.027 132 0.936 Cu-Pb 2.73 0.63 0.925 0.055 65 0.925 Adams-Bohart模型 (qe)exp/(mg·g-1) t0.5/min kAB/(10-3 L·mg-1 min-1) N0/(mg·L-1) R2 Cu-Zn 0.42 1003.38 0.558 1.36 125 Cu-Pb 0.68 518.46 0.776 0.59 70 -
[1] 宋明铭. 高碳粉煤灰综合利用技术研究[J]. 矿产综合利用, 2021(3):93-98. doi: 10.3969/j.issn.1000-6532.2021.03.015
SONG M M. Study on comprehensive utilization technology of high carbon fly ash[J]. Multipurpose Utilization of Mineral Resources, 2021(3):93-98. doi: 10.3969/j.issn.1000-6532.2021.03.015
[2] 李沛伦, 胡真, 王成行, 等. 酸改性粉煤灰的制备及其降解选矿废水COD研究[J]. 矿产综合利用, 2019(2):103-108. doi: 10.3969/j.issn.1000-6532.2019.02.021
LI P L, HU Z, WANG C X, et al. Experimental study on preparation of acid modified fly ash and its degradation of COD in mineral processing wastewater[J]. Multipurpose Utilization of Mineral Resources, 2019(2):103-108. doi: 10.3969/j.issn.1000-6532.2019.02.021
[3] 闫玉兵. 改性粉煤灰对含磷废水的处理研究进展[J]. 矿产综合利用, 2020(5):34-44. doi: 10.3969/j.issn.1000-6532.2020.05.004
YAN Y B. Research on modified fly ash treats for phosphorus wastewater[J]. Multipurpose Utilization of Mineral Resources, 2020(5):34-44. doi: 10.3969/j.issn.1000-6532.2020.05.004
[4] Huang X R, Zhao H H, Zhang G B, et al. Potential of removing Cd(Ⅱ) and Pb(Ⅱ) from contaminated water using a newly modified fly ash[J]. Chemosphere, 2020, 242:125148. doi: 10.1016/j.chemosphere.2019.125148
[5] 高宏, 李恒, 贺波, 等. 用改性粉煤灰微珠吸附处理铅锌硫化矿选矿废水[J]. 湿法冶金, 2018, 37(1):40-43.
GAO H, LI H, HE B, et al. Adsorption treatment of benefication wastewater of Pb-Zn sulfide ore by sulphuric acid modified fly ash[J]. Hydrometallurgy of China, 2018, 37(1):40-43.
[6] 黄训荣, 赵航航, 张贵宾, 等. 改性粉煤灰对废水中镉的吸附作用[J]. 应用生态学报, 2019, 30(9):3215-3223.
HUANG X R, ZHAO H H, ZHANG G B, et al. Adsorption of Cd2+ from wastewater by modified fly ash[J]. Chines Journal of Applied Ecology, 2019, 30(9):3215-3223.
[7] Zhang P Z, Zhang X X, Yuan X R, et al. Characteristics, adsorption behaviors, Cu(Ⅱ) adsorption mechanisms by cow manure biochar derived at various pyrolysis temperatures[J]. Bioresource Technology, 2021, 331:125013-125021. doi: 10.1016/j.biortech.2021.125013
[8] 骆欣, 杨怡心, 徐东耀. 热改性粉煤灰对水中Cu(Ⅱ)的吸附研究[J]. 应用化工, 2020, 49(9):2242-2251. doi: 10.3969/j.issn.1671-3206.2020.09.023
LUO X, YANG Y X, XU D Y. Study on adsorption of Cu(Ⅱ) by thermal modified fly ash[J]. Applied Chemical Industry, 2020, 49(9):2242-2251. doi: 10.3969/j.issn.1671-3206.2020.09.023
[9] 雷苏, 曾鹏鑫, 王鹏, 等. Na2CO3基吸附剂颗粒制备及其脱碳性能[J]. 化工进展, 2019, 38(8):3562-3571.
LEI S, ZENG P X, WANG P, et al. Investigation on granulation and CO2 uptake of Na2CO3-based sorbent pellets[J]. Chemical Industry and Engineering Progress, 2019, 38(8):3562-3571.
[10] Li Y, Liu S B, Wang C, et al. Effective column adsorption of triclosan from pure water and wastewater treatment plant effluent by using magnetic porous reduced graphene oxide[J]. Journal of Hazardous Materials, 2020, 386:121942.1-11.
[11] Hayati B, Maleki A, Najafi F, et al. Heavy metal adsorption using PAMAM/CNT nanocomposite from aqueous solution in batch and continuous fixed bed systems[J]. Chemical Engineering Journal, 2018, 346:258-270. doi: 10.1016/j.cej.2018.03.172
[12] Dorado A D, Gamisans X, Valderrama C, et al. Cr (Ⅲ) removal from aqueous solutions: a straightforward model approaching of the adsorption in a fixed-bed column[J]. Journal of Environmental Science and Health Part A, 2014, 49:179-186. doi: 10.1080/10934529.2013.838855
[13] Rokhsare G O, Behruz M, Ali N, et al. Competitive adsorption of nitrate in fixed-bed column packed with bio-inspired polydopamine coated zeolite[J]. Journal of Environmental Chemical Engineering, 2018, 6(2):2232-2240. doi: 10.1016/j.jece.2018.01.049
[14] Zhang X, Bai B, Puma G L, et al. Novel sea buckthorn biocarbon SBC@β-FeOOH composites: efficient removal of doxycycline in aqueous solution in a fixed-bed through synergistic adsorption and heterogeneous Fenton-like reaction [J]. Chemical Engineering Journal. 2016, 284: 698-707.
[15] 杜兆林, 陈洪安, 秦莉, 等. 纤维素基吸附材料除污工艺及吸附模型研究进展[J]. 农业资源与环境学报, 2020, 37(1):6-16.
DU Z L, CHEN H A, QIN L, et al. Decontamination and adsorption modelling by cellulose-based adsorption materials: A review[J]. Journal of Agricultural Resources and Environment, 2020, 37(1):6-16.
[16] He J, Zhou Q H, Guo J S, et al. Incredulity on assumptions for the simplified Bohart-Adams model: 17a-ethinylestradiol separation in lab-scale anthracite columns[J]. Journal of Hazardous Materials, 2020, 384:121501. doi: 10.1016/j.jhazmat.2019.121501
[17] Hui K S, Chao C Y H, Kot S C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash[J]. Journal of Hazardous Materials, 2005, 127(1-3):89-101. doi: 10.1016/j.jhazmat.2005.06.027
[18] Zhao D Z, Wang Z, Lu S, et al. An amidoxime-functionalized polypropylene fiber: Competitive removal of Cu(Ⅱ), Pb(Ⅱ) and Zn(Ⅱ) from wastewater and subsequent sequestration in cement mortar[J]. Journal of Cleaner Production, 2020, 274:123049. doi: 10.1016/j.jclepro.2020.123049
[19] Zhu Y, Hu J, Wang J. Competitive adsorption of Pb(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) onto xanthate-modified magnetic chitosan[J]. Journal of Hazardous Materials, 2012, 221-222:155-161. doi: 10.1016/j.jhazmat.2012.04.026
[20] Chen C, Wang J L. Influence of metal ionic characteristics on their biosorption capacity by Saccharomyces cerevisiae[J]. Applied Microbiology and Biotechnology, 2007, 74:911-917. doi: 10.1007/s00253-006-0739-1
-



 下载:
下载: