Application of TiO2-Montmorillonite Composite Carrier in Plate-type De-NOx Catalyst
-
摘要:
为提升平板式脱硝催化剂性能,以TiO2-蒙脱土、TiO2-酸改性蒙脱土为复合载体进行催化剂的制备。采用XRF、XRD、N2-吸附脱附、拉曼光谱、H2-TPR、NH3-TPD等表征手段对催化剂的物理化学性能进行分析。结果显示:与传统V2O5-MoO3/TiO2催化剂相比,采用上述复合载体制备的脱硝催化剂具有更高的比表面积和耐磨强度。V2O5-MoO3/TiO2-蒙脱土催化剂中碱金属元素(Na、K)的存在,降低了催化剂的还原性能和酸性性能,对催化剂的脱硝活性有负面影响。相比蒙脱土,酸改性蒙脱土的比表面积较高,碱金属元素含量降低,所以V2O5-MoO3/TiO2-酸改性蒙脱土催化剂的脱硝活性较高。此外,该催化剂还具备优良的抗SO2、H2O性能。
Abstract:In order to improve the catalytic property of the plate-type De-NOx catalysts, TiO2-montmorillonite and TiO2-acid treated montmorillonite were used as the composite carriers for the preparation of the catalyst. XRF, XRD, N2-adsorption, Raman, H2-TPR and NH3-TPD analysis were carried out to investigate the physical and chemical properties of the catalysts. The result shows that the BET surface area and the attrition strength of the catalysts with the using of composite carriers are higher than that of the traditional V2O5-MoO3/TiO2 catalyst. Alkali metal element (Na, K) contained in the montmorillonite has negative effect on the reducibility and acidity of V2O5-MoO3/TiO2-montmorillonite catalyst, which is unfavorable for the catalytic performance. The acid treatment improves the BET surface area of the montmorillonite, and decreases the alkali metal element content simultaneously. As a result, the catalytic activity of V2O5-MoO3/TiO2-acid treated montmorillonite catalyst is relatively high. Addtionally, this catalyst also exhibits well SO2 and H2O resistance.
-
Key words:
- De-NOx /
- Montmorillonite /
- Composite carrier /
- Plate-type
-

-
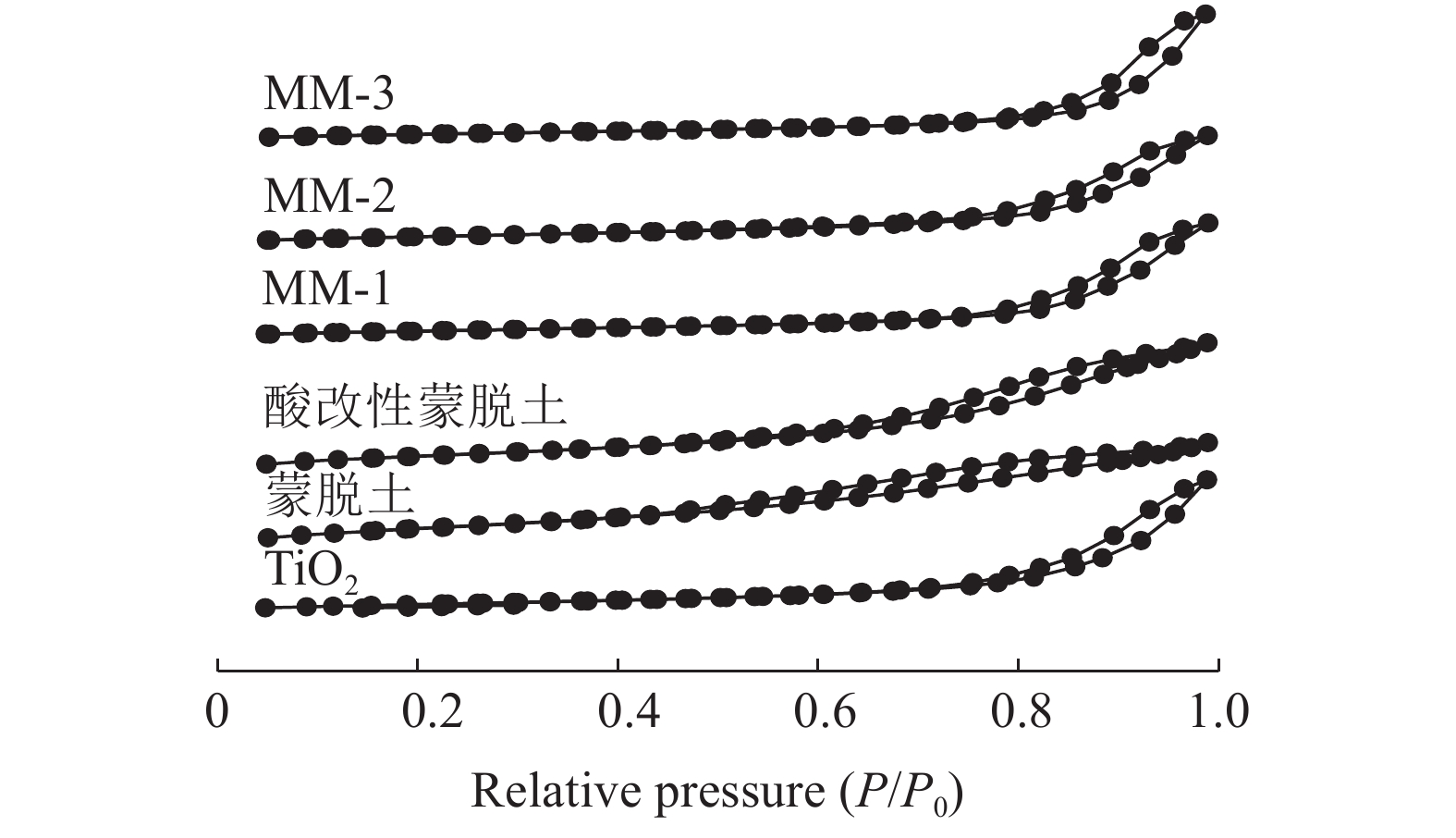
表 1 不同催化剂的XRF分析结果/%
Table 1. XRF results for the different catalysts
催化剂 V2O5 MoO3 Na2O K2O MM-1 1.43 2.89 0.07 0.01 MM-2 1.45 2.86 0.11 0.21 MM-3 1.42 2.88 0.08 0.09 表 2 不同催化剂的孔结构分析数据和机械性能
Table 2. Textural properties and attrition strength of the different catalysts
催化剂 比表面积/
(m2·g-1)孔容/
(cm3·g-1)平均孔径/
nm耐磨强度/
(mg·100 r-1)TiO2 82.43 0.361 17.24 - 蒙脱土 210.22 0.364 8.09 - 酸改性
蒙脱土253.48 0.425 5.74 - MM-1 76.01 0.312 17.61 74.8 MM-2 95.91 0.339 14.26 55.4 MM-3 97.56 0.348 14.15 54.9 -
[1] Liu Z S, Yu F, Ma C H, et al. A critical review of recent progress and perspective in practical denitration application[J]. Catalysis, 2019, 9(9):771-810.
[2] 王康, 朱林, 吴碧君, 等. SO2对燃煤电厂选择催化还原脱硝催化剂性能的影响[J]. 科学技术与工程, 2018, 18(13):323-327. doi: 10.3969/j.issn.1671-1815.2018.13.054
WANG K, ZHU L, WU B J, et al. The effect of SO2 on the performance of selective catalytic reduction denitrification catalysts for coal-fired power plants[J]. Science Technology and Engineering, 2018, 18(13):323-327. doi: 10.3969/j.issn.1671-1815.2018.13.054
[3] 李鹏, 张亚平, 肖睿, 等. 整体式V2O5-WO3/TiO2-ZrO2催化剂用于NH3选择性催化还原NOx[J]. 中南大学学报(自然科学版), 2013, 44(4):1719-1726.
LI P, ZHANG Y P, XIAO R. et al. Monolithic V2O5-WO3/TiO2-ZrO2 catalyst for selective catalytic reduction of NOx with NH3[J]. Journal of Central South University (Natural Science Edition), 2013, 44(4):1719-1726.
[4] 朱孝强, 黄亚继, 沈凯, 等. ZrO2掺杂的V2O5/TiO2催化剂表征及催化还原NOx[J]. 环境化学, 2012, 31(4):443-449.
ZHU X Q, HUANG Y J, SHEN K, et al. Characterization of ZrO2 doped V2O5/TiO2 catalyst and catalytic reduction of NOx[J]. Environmental Chemistry, 2012, 31(4):443-449.
[5] 喻明娥, 李彩亭, 王琰, 等. 以改性TiO2-SnO2为载体的SCR脱硝催化剂性能[J]. 环境工程学报, 2016, 10(7):3733-3738. doi: 10.12030/j.cjee.201502049
YU M E, LI C T, WANG Y, et al. Performance of SCR denitration catalyst supported by modified TiO2-SnO2[J]. Environmental Engineering Journal, 2016, 10(7):3733-3738. doi: 10.12030/j.cjee.201502049
[6] 马晓宇, 梁雨, 崔素萍, 等. 稻壳灰制备TiO2-SiO2复合载体脱硝催化材料[J]. 材料导报B:研究篇, 2018, 32(11):3984-3988.
MA X Y, LIANG Y, CUI S P, et al. Preparation of TiO2-SiO2 composite carrier denitrification catalytic material from rice husk ash[J]. Materials Review B:Research, 2018, 32(11):3984-3988.
[7] 马腾坤, 孔晓华, 房晶瑞, 等. Mn-Ce/TiO2催化剂载体掺杂非矿材料改性对其脱硝活性的影响[J]. 环境工程, 2019, 37(6):1-4.
MA T K, KONG X H, FANG J R, et al. Effect of modification of Mn-Ce/TiO2 catalyst carrier with non-mineral materials on its denitration activity[J]. Environmental Engineering, 2019, 37(6):1-4.
[8] 李宝茹, 李政, 安霞, 等. 酸改性蒙脱土负载杂多酸的制备及其催化乙醇制乙烯[J]. 太原理工大学学报, 2017, 48(1):30-35. doi: 10.16355/j.cnki.issn1007-9432tyut.2017.01.005
LI B R, LI Z, AN X, et al. Preparation of acid-modified montmorillonite-supported heteropolyacid and its catalysis for the production of ethylene from ethanol[J]. Journal of Taiyuan University of Technology, 2017, 48(1):30-35. doi: 10.16355/j.cnki.issn1007-9432tyut.2017.01.005
[9] 晁晶迪, 何洪, 宋丽云, 等. Pr掺杂对V2O5-MoO3/TiO2催化剂NH3-SCR反应活性的影响[J]. 高等学校化学学报, 2015, 36(3):523-530.
CHOU J D, HE H, SONG L Y, et al. Effect of Pr doping on the activity of V V2O5-MoO3/TiO2 catalyst NH3-SCR[J]. Chemical Journal of Chinese Universities, 2015, 36(3):523-530.
[10] Okada K, Arimitsu N, Kameshima Y, et al. Solid acidity 2: 1 type clay minerals activated by selective leaching[J]. Applied Clay Science, 2006, 31:185-193. doi: 10.1016/j.clay.2005.10.014
[11] 黄贵秋, 颜曦明, 钟书明, 等. 酸活化蒙脱土催化乙二胺合成哌嗪和三乙烯二胺[J]. 石油化工, 2018, 47(12):1338-1344. doi: 10.3969/j.issn.1000-8144.2018.12.007
HUANG G Q, YAN X M, ZHONG S M, et al. Acid-activated montmorillonite catalyzed the synthesis of piperazine and triethylenediamine from ethylenediamine[J]. Petrochemical Industry, 2018, 47(12):1338-1344. doi: 10.3969/j.issn.1000-8144.2018.12.007
[12] Choo S T, Lee Y G, Nam I S, et al. Characteristics of V2O5 supported on sulfated TiO2 for selective catalytic reduction of NO by NH3[J]. Applied Catalysis A: General 2000, 200: 177-188
[13] Dong G J, Bai Y, Zhang Y F, et al. Effect of the V4+(3+)/V5+ ration on the denitration activity for V2O5-WO3/TiO2 catalysts[J]. New Journal of Chemistry, 2015, 39:3588-3596. doi: 10.1039/C5NJ00015G
[14] Qiu Y, Liu B, Du J, et al. The monolithic cordierite supported V2O5–MoO3/TiO2 catalyst for NH3-SCR[J]. Chemical Engineering Journal, 2016, 294:264-272. doi: 10.1016/j.cej.2016.02.094
[15] Yu W C, Wu X D, Si Z C, et al. Influences of impregnation procedure on the SCR activity and alkali resistance of V2O5-WO3/TiO2 catalyst[J]. Applied Surface Science, 2013, 283:209-214. doi: 10.1016/j.apsusc.2013.06.083
[16] Laura C, Luca L, Isbella N, et al. SCR of NO by NH3 over TiO2-supported V2O5-MoO3 catalysts: reactivity and redox behavior[J]. Applied Catalysis B:Environmental, 1999, 22(1):63-77. doi: 10.1016/S0926-3373(99)00035-1
[17] Tang F S, Zhuang K, Yang F, et al. Effect of dispersion state and surface properties of supported vanadia on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of NO by NH3[J]. Chinese Journal of Catalysis, 2012, 33(6):933-940.
[18] Du X S, Gao X, Qiu K Z, et al. The reaction of poisonous alkali oxide with vanadia SCR catalyst and the afterward influence: A DFT and experimental study[J]. The Journal of Physical Chemistry, 2015, 119(4):1905-1912.
[19] Wallis P J, Gates W P, Patti A F, et al. Assessing and improving the catalytic activity of K-10 montmorillonite[J]. Green Chemistry, 2007, 9:980-986. doi: 10.1039/b701504f
[20] Huang L, Zong Y H, Wang H, et al. Influence of calcination temperature on the plate-type V2O5-MoO3/TiO2 catalyst for selective catalytic reduction of NO[J]. Reaction Kinetics, Mechanisms and Catalysis, 2018, 124:603-617. doi: 10.1007/s11144-018-1378-0
[21] 贾 勇, 周 军, 柏家串, 等. 砷、钾复合中毒选择性催化还原脱硝催化剂的再生[J]. 硅酸盐学报, 2016, 44(7):1025-1032. doi: 10.14062/j.issn.0454-5648.2016.07.18
JIA Y, ZHOU J, BAI J C, et al. Regeneration of selective catalytic reduction denitrification catalyst with arsenic and potassium compound poisoning[J]. Journal of The Chinese Ceramic Society, 2016, 44(7):1025-1032. doi: 10.14062/j.issn.0454-5648.2016.07.18
[22] Topsøe N Y, Topsøe H, Dumesic J A. Vanadia/titania catalysts for selective catalytic reduction (SCR) of nitric oxide by ammonia Ⅰ. Combined temperature programmed in situ FTIR and on-line mass spectroscopy studies[J]. Journal of Catalysis, 1995, 151(1):226-240. doi: 10.1006/jcat.1995.1024
-




 下载:
下载: