Effect of Temperature on the Co-production of Copper Slag and Middle Low-grade Phosphate Rock
-
摘要:
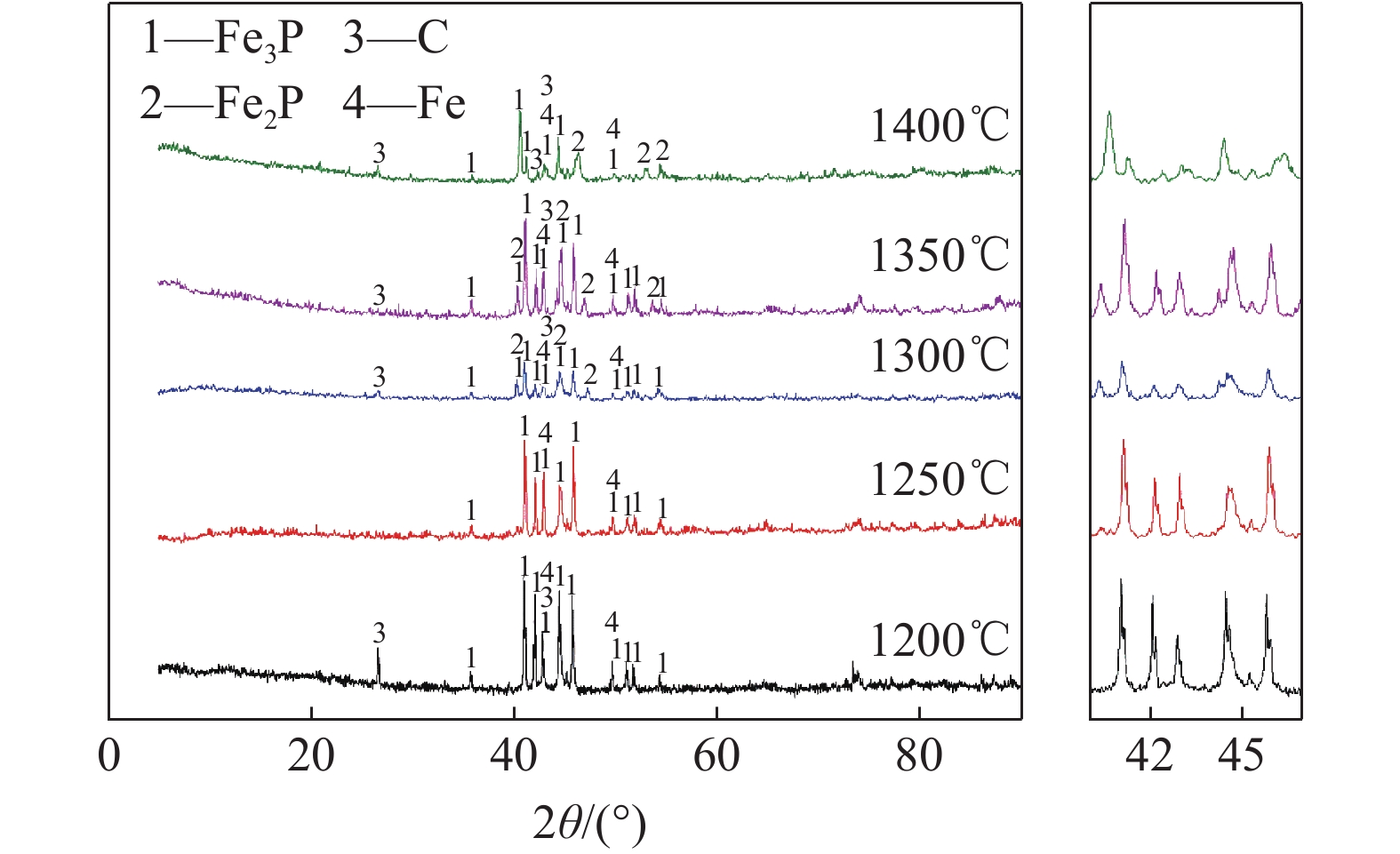
磷铁是一种重要的工业合金物,在炼钢、化工业及新能源领域和建筑业具有举足轻重的作用,磷铁的开发利用不仅可以为企业带来经济效益,也可为国家经济的发展注入活力。本文在T=1200~1400℃,R(CaO/SiO2)=1.0,t=60 min,C=12%条件下进行实验。以磷矿和铜渣为原料,石墨作还原剂,在高温节能管式炉中还原焙烧制取磷铁,还原后的试样进行分离、磨矿,采用XRD、SEM和EDS对磷铁及渣进行表征,结果显示:T<1300℃所得磷铁主要物相为Fe3P,T>1300℃所得磷铁主要物相为Fe3P、Fe2P。还原后测得渣的主要成分为硅酸盐类,以偏硅酸钙为主(CaSiO3)和含有少量的硅酸铝。
Abstract:Ferro-phosphorus is an important industrial alloy, which plays a pivotal role in steelmaking, chemical industry, new energy and construction industry. The development and utilization of ferro-phosphorus can not only bring economic benefits to enterprises, but also contribute to the development of national economy. In this paper, the experiment was carried out under the conditions of T=1200℃-1400℃, R(CaO/SiO2)=1.0, t=60 min and C=12%. Phosphate rock and copper slag were used as raw materials, graphite was used as reducing agent, and ferrophosphorus was produced by reduction and roasting in a high-temperature energy-saving tubular furnace. The reduced samples were separated and ground, XRD, SEM and EDS were used to characterize ferrophosphorus and slag. The results showed that the main phase of ferrophosphorus obtained is Fe3P at T<1300℃, and the main phase of ferrophosphorus obtained is Fe3P and Fe2P at T>1300℃. After reduction, it was measured that the main component of the slag was silicate, mainly calcium metasilicate (CaSiO3) and a small amount of aluminum silicate.
-
Key words:
- Ferro-phosphorus /
- Phosphate rock /
- Copper slag /
- High temperature reduction
-

-
表 1 磷矿和铜渣的化学成分/%
Table 1. Chemical composition of copper slag and phosphate ore
名称 Fe2O3 SiO2 Na2O SO3 MgO CaO Al2O3 P2O5 ZnO F 磷矿 1.06 9.79 0.43 0.85 1.70 48.94 1.38 32.98 0 2.87 铜渣 56.83 20.62 6.48 4.80 2.63 2.43 2.13 0.29 3.79 0 表 2 不同温度下点扫描结果/%
Table 2. Spot scan results at different temperatures
Fe P C O Ca Si Mg Al 1200(a) 82.02 14.37 2.70 0.79 0.00 0.00 0.05 0.07 1300(a) 83.22 12.91 2.74 0.72 0.00 0.40 0.01 0.00 1350(a) 82.86 13.34 2.52 0.67 0.07 0.52 0.03 0.00 1400(a) 82.89 12.58 2.96 0.93 0.08 0.45 0.03 0.09 1200(b) 93.42 0.91 4.89 0.65 0.00 0.00 0.06 0.08 1300(b) 87.91 0.62 6.49 1.01 0.00 3.84 0.07 0.06 1350(b) 79.04 0.30 13.04 4.84 0.08 2.55 0.15 0.00 1400(b) 82.89 0.31 15.14 0.68 0.09 0.62 0.00 0.01 1200(c) 43.46 0.56 53.29 2.49 0.04 0.00 0.09 0.07 1300(c) 75.90 1.65 19.67 1.26 0.00 1.31 0.17 0.05 1350(c) 6.56 0.06 89.07 4.05 0.11 0.11 0.02 0.02 1400(c) 10.53 0.07 81.32 7.59 0.24 0.22 0.03 0.00 1200(d) 47.87 0.95 15.67 34.56 0.17 0.34 0.24 0.19 1300(d) 40.70 2.36 33.99 19.88 0.00 1.36 1.27 0.45 1350(d) 48.37 12.18 17.33 20.55 0.26 0.53 0.16 0.61 1400(d) 69.72 12.09 13.45 4.07 0.09 0.38 0.05 0.14 -
[1] 王富龙, 王珏, 周骏宏, 等. 磷铁的酸解反应活性试验研究[J]. 湿法冶金, 2020, 39(1):26-29. doi: 10.13355/j.cnki.sfyj.2020.01.006
WANG F L, WANG J, ZHOU J H, et al. Research on acidolysis reaction activity of iron phosphorus[J]. Hydrometallurgy, 2020, 39(1):26-29. doi: 10.13355/j.cnki.sfyj.2020.01.006
[2] 马毅, 沈文喆, 袁梅梅, 等. 磷铁渣制备电池级纳米磷酸铁[J]. 化工进展, 2019, 38(11):5015-5023. doi: 10.16085/j.issn.1000-6613.2019-0049
MA Y, SHENG W Z, YUAN M M, et al. Preparation of battery-sized iron phosphate from iron phosphate slag[J]. Chemical Progress, 2019, 38(11):5015-5023. doi: 10.16085/j.issn.1000-6613.2019-0049
[3] 赵曼, 肖仁贵, 廖霞, 等. 水热法以磷铁制备电池级磷酸铁的研究[J]. 材料导报, 2017, 31(10):25-31. doi: 10.11896/j.issn.1005-023X.2017.010.006
ZHAO M, XIAO R G, LIAO X, et al. Study on the preparation of battery-grade iron phosphate by hydrothermal method[J]. Material Guide, 2017, 31(10):25-31. doi: 10.11896/j.issn.1005-023X.2017.010.006
[4] Yersak T A, Evans T, Whiteley J M, et al. Derivation of an iron pyrite all-solid-state composite electrode with ferrophosphorus, sulfur, and lithium sulfide as precursors[J]. Journal of the Electrochemical Society, 2014, 161(5):663-667.
[5] 王涛, 付磊, 李宁. 某硅钙质胶磷矿正反浮选试验研究[J]. 矿产综合利用, 2020(2):91-95. doi: 10.3969/j.issn.1000-6532.2020.02.016
WANG T, FU L, LI N. Study on direct-reverse flotation of a silica calcinate phosphate ore[J]. Multipurpose Utilization of Mineral Resources, 2020(2):91-95. doi: 10.3969/j.issn.1000-6532.2020.02.016
[6] 吴中贤, 姜效军, 陶东平. 新型胶磷矿反浮选脱硅阳离子捕收剂试验研究[J]. 矿产综合利用, 2020(5):115-119. doi: 10.3969/j.issn.1000-6532.2020.05.017
WU Z X, JIANG X J, TAO D P. Experimental study on a novel cationic collector for reverse flotation of collophane for silica removal[J]. Multipurpose Utilization of Mineral Resources, 2020(5):115-119. doi: 10.3969/j.issn.1000-6532.2020.05.017
[7] 丁银贵, 薛逊, 倪文, 等. 铜渣直接还原-磨选二次尾矿利用方式研究[J]. 矿产综合利用, 2019(3):133-140. doi: 10.3969/j.issn.1000-6532.2019.03.030
DING Y G, XUE X, NI W, et al. Research on utilization of tailings from copper slag through RHF direct reduction-grinding separation process[J]. Multipurpose Utilization of Mineral Resources, 2019(3):133-140. doi: 10.3969/j.issn.1000-6532.2019.03.030
[8] 李涛, 刘晨, 佘世杰. 铜渣中铁铜回收的试验研究[J]. 矿产综合利用, 2020(2):145-150. doi: 10.3969/j.issn.1000-6532.2020.02.026
LI T, LIU C, SHE S J. Research on recovery of iron and copper in copper slag[J]. Multipurpose Utilization of Mineral Resources, 2020(2):145-150. doi: 10.3969/j.issn.1000-6532.2020.02.026
[9] 白倩. 磷铁真空热分解及磷铁加镍制备金属磷化物[D]. 昆明: 云南师范大学, 2016.
BAI Q. Preparation of metal phosphides by vacuum thermal decomposition of iron phosphate and nickel[D]. Kunming: Yunnan Normal University, 2016.
[10] SUN Y, CHEN M, Balladares E, et al. Effect of CaO on the liquid/spinel/matte/gas equilibria in the Si-Fe-O-Cu-S system at controlled P(SO2) 0.3 and 0.6 atm[J]. Calphad, 2020:69.
[11] 李洪桂. 冶金原理[M]. 北京: 科学出版社, 2005.
LI H G. Metallurgical principle[M]. Beijing: Science Press, 2005.
[12] GUANG H L, MING J R, CHONG Z O, et al. Distribution characteristics of phosphorus in the metallic iron during solid-state reductive roasting of oolitic hematite ore[J]. ISIJ International, 2015, 55(11).
[13] Prince S, Avimany D, Gary W, et al. Recovery of metal values from copper slag and reuse of residual secondary slag[J]. Waste Management, 2017:70.
-




 下载:
下载: