Research Progress on the Interaction Mechanism between Hyperaccumulator and Heavy Metals and Its Application
-
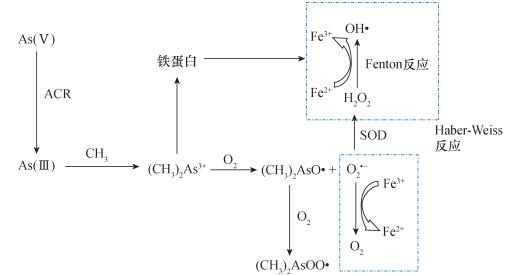
摘要: 社会发展过程中对矿产资源的勘查和开采利用所带来的重金属污染已对生态系统和人类健康造成严重威胁。超富集植物对重金属具有超富集、超耐受能力,是降低环境重金属污染、保障人类健康、实现绿色矿产勘查的有效途径,在植物修复、植物采矿和植物找矿中已获得了广泛应用。深入探索超富集植物的富集和耐受机制,揭示重金属-植物相互作用规律,提高植物对重金属的富集能力,是当前国际上研究热点。本文在简要介绍重金属对植物作用的基础上,阐述了重金属诱导氧化应激机制,重点关注重金属超富集植物富集机理研究,对其在解毒和耐受机制等领域的研究进展进行了评述。当前研究认为:①对超富集植物而言,根系分泌物与根际微生物的共同作用促进了重金属溶解,经共质体、质外体途径吸收后,重金属通过木质部向上转运,并隔离在液泡中,实现对重金属的超富集;②重金属通过与小分子有机酸、细胞壁、植物螯合肽结合,以及液泡隔离,可降低细胞质中游离金属离子浓度,增强植物耐受性;③重金属胁迫下,植物将激活多种特异性抗氧化酶,抵御氧化应激反应,实现对重金属的超耐受。④本文分析认为,植物中砷诱导的氧化应激反应机制可能是由砷的还原与甲基化过程及Haber-Weiss反应三部分构成。对重金属超富集植物的富集与耐受过程所涉及的生理与生化作用进行深入研究,揭示关键性影响因素与相关规律,寻找提升其特异性富集与指示能力的有效途径,将有助于超富集植物研究与应用向纵深发展。Abstract:
BACKGROUND Heavy metal pollution caused by the exploitation of mineral resources in the social development has caused serious threats to the ecosystem and human health. Hyperaccumulating plants have super-enrichment and super-tolerance capabilities for heavy metals, which is an effective way to reduce environmental heavy metal pollution, protect human health, and realize green mineral exploration. It has been widely used in phytoremediation, plant mining and plant prospecting. OBJECTIVES To better understand the enrichment and tolerance mechanisms of hyperaccumulating plants, reveal the principles of heavy metal-plant interactions, and improve the ability of plants to accumulate heavy metals. METHODS Based on a brief description of the effects of heavy metals on plants, the focus of this article is the accumulation mechanism of heavy metal hyperaccumulation plants, and a review of the progress in the fields of detoxification and tolerance mechanisms. RESULTS (1) The root exudates of hyperaccumulator and microorganisms work together to promote the dissolution of heavy metals. After being absorbed by the symplastic and apoplastic pathways, the heavy metals are transported upwards to aerial parts through xylem, and segregated in vacuoles, achieving the hyperaccumulation of heavy metals. (2) Concentration of free metal ions in cytoplasm can be reduced by combining heavy metals with small molecular organic acids, cell walls, phytochelatins and vacuole isolation, which increases plant tolerance. (3) Under heavy metal stress, plants activate a variety of specific antioxidant enzymes to resist oxidative stress and achieve hypertolerance on heavy metals. (4) A possible mechanism is suggested that arsenic-induced oxidative stress in plants should be composed of arsenic reduction and methylation, and Haber-Weiss reaction. CONCLUSIONS In-depth research on the physiological and biochemical processes involved in hyperaccumulation and hypertolerance of hyperaccumulator reveals key factors and related principles. Finding effective ways to improve their specific accumulation and indication capabilities will contribute to the research and application of hyperaccumulators to develop in depth. -

-
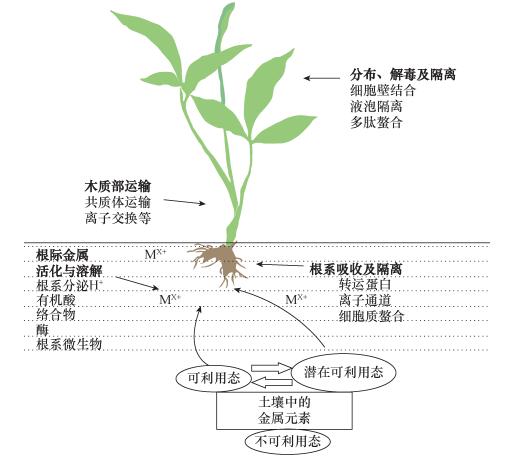
图 2 植物体内重金属元素超富集的主要过程与机制[50]
Figure 2.
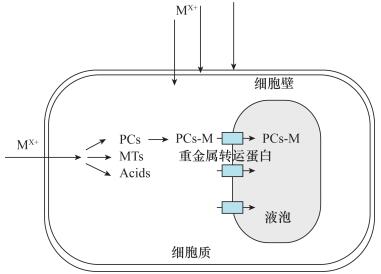
图 3 重金属液泡积累机制,液泡膜转运蛋白直接将重金属元素带入液泡[99]
Figure 3.
表 1 植物中微量元素的作用与机制
Table 1. Function and mechanism of micronutrients in plants
微量元素 作用(适量) 过量
(干重浓度)毒性机制 毒性症状 Zn[13] 促进植物形成花粉;氧化还原酶、转移酶、
水解酶、裂解酶、异构酶、连接酶组成成分>20mg/kg 增强脂质过氧化酶活性 引起遗传变异,抑制植物
生长Cu[13] 多种酶辅因子;参与细胞壁代谢,叶绿体、
线粒体电子传递;氧化磷酸化和铁动员,
氮同化,脱落酸合成等>20μg/g 抑制酶活性及蛋白质
功能,引起氧化应激抑制胚芽发育、种子活性、
植株发育,导致萎黄、坏死Mn[13] 参与ATP酰基脂质、蛋白质、脂肪酸生物
合成,参与RuBP羧化酶反应,光合作用
的氧化还原过程>(10~100)μg/g 干扰其他营养元素吸收
利用,诱导氧化应激影响能量代谢,降低光合
作用速率Se[24, 28] 保护细胞膜,防止不饱和脂肪酸氧化 >2mg/kg 非特异性硒蛋白积累,
氧化应激抑制植物生长 Ni[29-30] 脲酶组成成分 >(10~50)mg/kg 破坏叶绿素结构,降低
叶绿素含量抑制光合作用、氮代谢、
酶活性,产生活性氧,生长
速率下降Co[30] 诱导淀粉积累 >368mg/kg 破坏叶绿素结构,降低
叶绿素含量降低生长速率,光合作用
速率下降表 2 在不同培养条件下生长的不同植物中,重金属激活抗氧化酶差异
Table 2. Heavy metal-induced activation of antioxidant enzymes in different plant species grown in different condition
植物名称 胁迫元素与浓度 胁迫时间 基质 抗氧化酶 活性变化情况 江南星厥[109]
(Microsorum fortunei)Cd:1000μmol/L 15d 水培 CAT、POD、GST、CCP 降低 水合欢[110]
(Neptunia olerace)Cd:50、100、180mg/kg Pb:500、1000、1800mg/kg 37d 土培 根中CAT、茎中SOD、叶中POD 先降低后升高 根和茎中SOD、茎中CAT、叶中POD 大聚藻[111]
(Myriophyllum aquaticum)Cd:10、20、40、80、160mg/kg 28d 水培 SOD、POD、PRO 升高 印度芥菜[112] Cu、Zn、Pb、Cd:50μmol/L 96h 水培 SOD、CAT、APX 根部升高,地上部分先升高后降低(高于对照组) 墨旱莲[113]
(Eclipta prostrata)Pb:100、200、400、800、1600mg/kg 30d 土培 SOD、CAT、APX、GR 升高 印度芥菜、紫花苜蓿[114] Cd:7 5、150、300、600mg/kg 14d 土培 SOD 紫花苜蓿茎中升高 CAT 两者根、茎中均有升高 注:CAT—过氧化氢酶(catalase);POD—过氧化物酶(peroxidase);GST—谷胱甘肽硫转移酶(glutathione-S-transferase);CCP—细胞色素c过氧化物酶(cytochrome c peroxidase);SOD—超氧化物歧化酶(superoxide dismutase);PRO—脯氨酸(proline);APX—抗坏血酸过氧化物酶(ascorbate peroxidase);GR—谷胱甘肽还原酶(glutathione reductase)。 表 3 部分重金属超富集植物及其富集能力和应用
Table 3. Accumulation ability of several hyperaccumulators and their application
元素 超富集植物名称 生长条件 植物中重金属浓度(干重) 应用 Ni 褐蓝菜[75] 100μmol/L水培,>60天 根:33.6μmol/g
叶:130μmol/g/ Odontarrhena chalcidica
(庭芥属)[116]蛇纹石(serpentine)土壤,6.5个月 地上部分:13μg/g 植物采矿 小野芥菜[116]
(Noccaea goesingensis)蛇纹石土壤,11个月 地上部分:8μg/g 植物采矿 Senecio conrathii(菊科)[117] 总Ni:503μg/g,可溶性
Ni:0.1μg/g,土壤叶:1558μg/g 植物修复 髭脉桤叶树[118]
(Clethra barbinervis)500μmol/L水培,12周 根:1310μg/g
茎:542μg/g
叶:804μg/g/ 少根紫萍[30]
(Landoltia punctata)10mg/L水培,10天 叶:2013mg/kg 植物修复 Co 髭脉桤叶树[118] 500μmol/L水培,12周 根:1810μg/g
茎:246μg/g
叶:1770μg/g/ 少根紫萍[30] 10mg/L水培,10天 叶:1998mg/kg 植物修复 Cd 伴矿景天[82] 总Cd:36~157mg/kg,
CaCl2可提取态Cd:
1.83~14.2mg/kg,土壤地上部分:574~1470mg/kg 植物修复 多穗稗[65] 100mg/L水培,62天 根:299mg/kg
叶:233mg/kg植物修复 东南景天[79] 25μmol/L水培,30天 根:150μg/g;
地上部分:500μg/g植物修复 宝山堇菜[119]
(Viola baoshanensis)100μmol/L水培,2天 根:3500mg/kg
地上部分:1750mg/kg植物修复 狐尾藻[111]
(Myriophyllum aquaticum)40mg/L水培,28天 17970mg/kg 植物修复 龙葵[66] 20mg/kg,土壤,14天 根:95μg/g
地上部分:128μg/g植物修复 Zn 褐蓝菜[75] 100μmol/L水培,60天 根:46.4μmol/g
叶:161μmol/g(干重)/ 伴矿景天[82] 总Zn:1930~6200mg/kg,
CaCl2可提取态
Zn:24~162mg/kg,土壤地上部分:9020~14600mg/kg 植物修复 As 凤尾蕨[120] 10mg/kg,土壤,30天 根:1885mg/kg
叶:2562mg/kg植物修复 Pb 墨旱莲[113]
(Eclipta prostrata)1600mg/kg,土壤,30天 根:7229μg/g
地上部分:12484μg/g植物修复 -
[1] Jiang P, Hou Z G, Bolin J M, et al.RNA-Seq of human neural progenitor cells exposed to lead (Pb) reveals transcriptome dynamics, splicing alterations and disease risk associations[J].Toxicological Sciences, 2017, 159(1):251-265. doi: 10.1093/toxsci/kfx129
[2] Moynihan M, Peterson K E, Cantoral A, et al.Dietary predictors of urinary cadmium among pregnant women and children[J].Science of the Total Environment, 2017, 575:1255-1262. doi: 10.1016/j.scitotenv.2016.09.204
[3] Lintern M, Anand R, Ryan C, et al.Natural gold particles in Eucalyptus leaves and their relevance to exploration for buried gold deposits[J].Nature Communications, 2013, 4(4):2614. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=aec86ceeb26cae2fedad81514fb248d4
[4] Vardanyan N, Sevoyan G, Navasardyan T, et al.Recovery of valuable metals from polymetallic mine tailings by natural microbial consortium[J].Environmental Technology, 2019, 40(26):3467-3472. doi: 10.1080/09593330.2018.1478454
[5] 袁玲, 孟扬, 左玉明.黄金矿山尾矿资源回收和综合利用[J].黄金, 2010, 31(2):52-56. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=huangj201002014
Yuan L, Meng Y, Zuo Y M.Recovery and comprehensive utilization of gold tailings[J].Gold, 2010, 31(2):52-56. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=huangj201002014
[6] Baker A J M.Accumulators and excluders-strategies in the response of plants to heavy metals[J].Journal of Plant Nutrition, 1981, 3(1-4):643-654. doi: 10.1080/01904168109362867
[7] Krämer U.Metal hyperaccumulation in plants[J].Annual Review of Plant Biology, 2010, 61:517-534. doi: 10.1146/annurev-arplant-042809-112156
[8] Kumar S S, Malyan S K, Kadier A, et al.Phytoremediation and rhizoremediation: Uptake, mobilization and sequestration of heavy metals by plants[M]//Singh D P, Singh H B, Prabha R.Plant-microbe interactions in agro-ecological perspectives: Volume 2: Microbial interactions and agro-ecological impacts.Singapore: Springer, 2017: 367-394.
[9] Sheoran V, Sheoran A S, Poonia P.Phytomining of gold:A review[J].Journal of Geochemical Exploration, 2013, 128:42-50. doi: 10.1016/j.gexplo.2013.01.008
[10] Lintern M J, Anand R R.Dispersion of gold and other metals by trees, gravels and soils near Boddington gold deposit, western Australia[J].Journal of Geochemical Exploration, 2017, 181:10-21. doi: 10.1016/j.gexplo.2017.06.016
[11] Harguinteguy C A, Cofré M N, Cirelli A F, et al.The macrophytes Potamogeton pusillus L. and Myriophyllum aquaticum (Vell.) Verdc. as potential bioindicators of a river contaminated by heavy metals[J].Microchemical Journal, 2016, 124:228-234. doi: 10.1016/j.microc.2015.08.014
[12] Hakeem K, Rehman K.Crop production and global environmental issues[M]//Shahid M, Khalid S, Abbas G, et al.Heavy metal stress and crop productivity.Cham: Springer, 2015.
[13] Tripathi D K, Singh S, Singh S, et al.Micronutrients and their diverse role in agricultural crops:Advances and future prospective[J].Acta Physiologiae Plantarum, 2015, 37:139. doi: 10.1007/s11738-015-1870-3
[14] Kopittke P M, Blamey F P C, Mckenna B A, et al.Toxicity of metals to roots of cowpea in relation to their binding strength[J].Environmental Toxicology and Chemistry, 2011, 30(8):1827-1833. doi: 10.1002/etc.557
[15] Gupta D, Corpas F, Palma J.Heavy metal stress in plants[M]//Gupta D K, Vandenhove H, Inouhe M.Role of phytochelatins in heavy metal stress and detoxification mechanisms in plants.Heidelberg: Springer, 2013.
[16] Whitacre D.Reviews of environmental contamination and toxicology volume 232[M]//Shahid M, Pourrut B, Dumat C, et al.Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants.Cham: Springer, 2014.
[17] Galloa M, Morseb D, Hollnagel H C, et al.Oxidative stress and toxicology of Cu2+ based on surface areas in mixed cultures of green alga and cyanobacteria:the pivotal role of H2O2[J].Aquatic Toxicology, 2020, 222:105450. doi: 10.1016/j.aquatox.2020.105450
[18] Jomova K, Valko M.Advances in metal-induced oxidative stress and human disease[J].Toxicology, 2011, 283(2-3):65-87. doi: 10.1016/j.tox.2011.03.001
[19] Thounaojam T C, Panda P, Mazumdar P, et al.Excess copper induced oxidative stress and response of antioxidants in rice[J].Plant Physiology and Biochemistry, 2012, 53:33-39. doi: 10.1016/j.plaphy.2012.01.006
[20] Küpper H, Andresen E.Mechanisms of metal toxicity in plants[J].Metallomics, 2016, 8(3):269-285. doi: 10.1039/C5MT00244C
[21] Santos E F, Santini J M K, Paixao A P, et al.Physiologica l highlights of manganese toxicity symptoms in soybean plants:Mn toxicity responses[J].Plant Physiology and Biochemistry, 2017, 113:6-19. doi: 10.1016/j.plaphy.2017.01.022
[22] Tavanti R F R, Queiroz G D, Silva A C D, et al.Changes in photosynthesis and antioxidant metabolism of cotton (Gossypium hirsutum L.) plants in response to manganese stress[J].Archives of Agronomy and Soil Science, 2019. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/03650340.2019.1637857
[23] Yu Y, Fu P N, Huang Q P, et al.Accumulation, sub-cellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages[J].Chemosphere, 2019, 216:331-340. doi: 10.1016/j.chemosphere.2018.10.138
[24] Hoewyk D V.A tale of two toxicities:Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants[J].Annals of Botany, 2013, 112(6):965-972. doi: 10.1093/aob/mct163
[25] Kaur M, Sharma S.Influence of selenite and selenate on growth, leaf physiology and antioxidant defense system in wheat (Triticum aestivum L.)[J].Journal of the Science of Food and Agriculture, 2018, 98(15):5700-5710. doi: 10.1002/jsfa.9117
[26] Knauert S, Knauer K.The role of reactive oxygen species in copper toxicity to two freshwater green algae[J].Journal of Phycology, 2010, 44(2):311-309. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1111/j.1529-8817.2008.00471.x
[27] Opdenakker K, Remans T, Keunen T, et al.Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels[J].Environmental and Experimental Botany, 2012, 83:53-61. doi: 10.1016/j.envexpbot.2012.04.003
[28] Kolbert Z, Lehotai N, Molnár A, et al."The roots" of selenium toxicity:A new concept[J].Plant Signaling & Behavior, 2016, 11(10):1241935. http://www.tandfonline.com/doi/full/10.1080/15592324.2016.1241935
[29] Pas L V D, Robert A I.Towards an understanding of the molecular basis of nickel hyperaccumulation in plants[J].Plants, 2019, 8:11. doi: 10.3390/plants8010011
[30] Guo L, Ding Y Q, Xu Y L, et al.Responses of Landoltia punctata to cobalt and nickel:Removal, growth, photosynthesis, antioxidant system and starch metabolism[J].Aquatic Toxicology, 2017, 190:87-93. doi: 10.1016/j.aquatox.2017.06.024
[31] Tan L L, Xue X G, Du J, et al.Probing the molecular toxic mechanism of lead(Ⅱ) ions with glutathione peroxidase 6 from Arabidopsis thaliana[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2020, 226:117597. doi: 10.1016/j.saa.2019.117597
[32] Abbas G, Murtaza B, Bibi I, et al.Arsenic uptake, toxicity, detoxification, and speciation in plants:Physiological, biochemical, and molecular aspects[J].International Journal of Environmental Research and Public Health, 2018, 15:59. doi: 10.3390/ijerph15010059
[33] Kehrer J P.The Haber-Weiss reaction and mechanisms of toxicity[J].Toxicology, 2000, 149(1):43-50. http://www.onacademic.com/detail/journal_1000034185137910_b550.html
[34] Kumar A, Prasad M N V.Plant-lead interactions:transport, toxicity, tolerance, and detoxification mechanisms[J].Ecotoxicology and Environmental Safety, 2018, 166:401-418. doi: 10.1016/j.ecoenv.2018.09.113
[35] Ugya A Y, Imam T S, Li A F, et al.Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production:A mini review[J].Chemistry and Ecology, 2020, 36(2):174-193. http://www.tandfonline.com/doi/abs/10.1080/02757540.2019.1688308
[36] Ghosh P, Rathinasabapathi B, Lena Q M. Phosphorus solubilization and plant growth enhancement by arsenic-resistant bacteria[J].Chemosphere, 2015, 134:1-6. doi: 10.1016/j.chemosphere.2015.03.048
[37] Kumar S, Dubey R S, Tripathi R D, et al.Omics and biotechnology of arsenic stress and detoxification in plants:Current updates and prospective[J].Environment International, 2015, 74:221-230. doi: 10.1016/j.envint.2014.10.019
[38] Tripathi R D, Tripathi P, Dwivedi S, et al.Arsenomics:Omics of arsenic metabolism in plants[J].Frontiers in Physiology, 2012, 3:275. http://europepmc.org/articles/PMC3429049/
[39] Swaran J S F.Arsenic-induced oxidative stress and its reversibility[J].Free Radical Biology & Medicine, 2011, 51(2):257-281. http://www.ncbi.nlm.nih.gov/pubmed/21554949
[40] Sharma I.Arsenic induced oxidative stress in plants[J].Biologia, 2012, 67(3):447-453. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.2478/s11756-012-0024-y
[41] Malik J A, Goel S, Kaur N, et al.Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms[J].Environmental and Experimental Botany, 2012, 77:242-248. doi: 10.1016/j.envexpbot.2011.12.001
[42] Tamás L, Zelinová V.Mitochondrial complex Ⅱ-deri-ved superoxide is the primary source of mercury toxicity in barley root tip[J].Journal of Plant Physiology, 2017, 209:68-75. doi: 10.1016/j.jplph.2016.10.014
[43] Chen Q, Zhang Z Y, Liu Y Y, et al.Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings[J].Plant Growth Regulation, 2017, 81(2):253-264. doi: 10.1007/s10725-016-0202-y
[44] Gupta D K, Pena B, Romero P M C, et al.NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity[J].Plant, Cell & Environment, 2017, 40(4):509-526. http://www.ncbi.nlm.nih.gov/pubmed/26765289
[45] Zhang H H, Li X, Xu Z S, et al.Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves:Photosynthetic function and reactive oxygen species (ROS) metabolism responses[J].Ecotoxicology and Environmental Safety, 2020, 195:110469. doi: 10.1016/j.ecoenv.2020.110469
[46] Mitch M L.Phytoextraction of toxic metals:A review of biological mechanisms[J].Journal of Environmental Quality, 2002, 31(1):109-120. http://europepmc.org/abstract/MED/11837415
[47] Fayigaa A O, Maa L Q, Cao X D, et al.Effects of heavy metals on growth and arsenic accumulation in the arsenic hyperaccumulator Pteris vittata L[J].Environmental Pollution, 2004, 132(2):289-296. doi: 10.1016/j.envpol.2004.04.020
[48] Rascio N, Izzo F N.Heavy metal hyperaccumulating plants:How and why do they do it? and what makes them so interesting?[J].Plant Science, 2011, 180:169-181. doi: 10.1016/j.plantsci.2010.08.016
[49] Freeman J L, Quinn C F, Lindblom S D, et al.Selenium protects the hyperaccumulator Stanleya pinnata against black-tailed prairie dog herbivory in native seleniferous habitats[J].American Journal of Botany, 2009, 96(6):1075-1085. doi: 10.3732/ajb.0800287
[50] Sheoran V, Sheoran A S, Poonia P.Phytomining:A review[J].Minerals Engineering, 2009, 22(12):1007-1019. doi: 10.1016/j.mineng.2009.04.001
[51] Li T Q, Tao Q, Liang C F, et al.Complexation with dissolved organic matter and mobility control of heavy metals in the rhizosphere of hyperaccumulator Sedum alfredii[J].Environmental Pollution, 2013, 182:248-255. doi: 10.1016/j.envpol.2013.07.025
[52] Salinitroa M, Entb A N D, Tognacchini A, et al.Stress responses and nickel and zinc accumulation in different accessions of Stellaria media (L.) Vill.in response to solution pH variation in hydroponic culture[J].Plant Physiology and Biochemistry, 2020, 148:133-141. doi: 10.1016/j.plaphy.2020.01.012
[53] Peng C, Xu C, Liu Q, et al.Fate and transformation of CuO nanoparticles in the soil-rice system during the life cycle of rice plants[J].Environmental Science & Technology, 2017, 51(9):4907-4917. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3a03e6f916726717f3a110e2db6fffbf
[54] Liu X, Fu J W, Guan D X, et al.Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata[J].Environmental Science & Technology, 2016, 50(17):9070-9077. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e844969c9b56ea80aee1cdf676f646e3
[55] Ge Y, Priester J H, Werfhorst L C V D, et al.Soybean plants modify metal oxide nanoparticle effects on soil bacterial communities[J].Environmental Science & Technology, 2014, 48(22):13489-13496. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=40a30aba396d9dc05ea443482f74ed99
[56] Ali H, Khan E, Sajad M A.Phytoremediation of heavy metals-Concepts and applications[J].Chemosphere, 2013, 91(7):869-881. doi: 10.1016/j.chemosphere.2013.01.075
[57] Mahrousa N N, Columbusa M P, Southam G, et al.Changes in microbial community structure and increased metal bioavailability in a metal-contaminated soil and in the rhizosphere of corn (Zea mays)[J].Rhizosphere, 2019, 11:100169. doi: 10.1016/j.rhisph.2019.100169
[58] 曾远, 罗立强.土壤中特异性微生物与重金属相互作用机制与应用研究进展[J].岩矿测试, 2017, 36(3):209-221. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201701170009
Zeng Y, Luo L Q.Research progress on the application and interaction mechanism between specific microorganisms and heavy metals in soil[J].Rock and Mineral Analysis, 2017, 36(3):209-221. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201701170009
[59] He H D, Ye Z H, Yang D J, et al.Characterization of endophytic Rahnella sp.JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus[J].Chemosphere, 2013, 90(6):1960-1965. doi: 10.1016/j.chemosphere.2012.10.057
[60] He B Y, Yu D P, Chen Y, et al.Use of low-calcium cultivars to reduce cadmium uptake and accumulation in edible amaranth (Amaranthus mangostanus L.)[J].Chemosphere, 2017, 171:588-594. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=90945c9a80906fe9bd334d6d5a7dc7ae
[61] Wu Z C, Zhao X H, Sun X C, et al.Xylem transport and gene expression play decisive roles in cadmium accumulation in shoots of two oilseed rape cultivars (Brassica napus)[J].Chemosphere, 2015, 119:1217-1223. doi: 10.1016/j.chemosphere.2014.09.099
[62] Tao Q, Jupa R, Luo J P, et al.The apoplasmic pathway via the root apex and lateral roots contributes to Cd hyperaccumulation in the hyperaccumulator Sedum alfredii[J].Journal of Experimental Botany, 2017, 68(3):739-751. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a418367952ea1179ba9bcaee10182fca
[63] Hendel A M, Zubko M, Karcz J, et al.Fate of neutral-charged gold nanoparticles in the roots of the Hordeum vulgare L.cultivar Karat[J].Scientific Reports, 2017, 7(1):3014. doi: 10.1038/s41598-017-02965-w
[64] Attwood T S, Unrine J M, Stone J W, et al.Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings[J].Nanotoxicology, 2012, 6(4):353-360. doi: 10.3109/17435390.2011.579631
[65] Solis D F A, Gonzalez C M C, Carrillo G R, et al. Accumulation and localization of cadmium in Echinochloa polystachya grown within a hydroponic system[J].Journal of Hazardous Materials, 2007, 141(3):630-636. doi: 10.1016/j.jhazmat.2006.07.014
[66] Bao T, Sun T H, Sun L.Low molecular weight organic acids in root exudates and cadmium accumulation in cadmium hyperaccumulator Solanum nigrum L.and nonhyperaccumulator Solanum lycopersicum L.[J].African Journal of Biotechnology, 2011, 10(75):17180-17185. http://www.researchgate.net/publication/272690006_Low_molecular_weight_organic_acids_in_root_exudates_and_cadmium_accumulation_in_cadmium_hyperaccumulator_Solanum_nigrum_L._and_non-hyperaccumulator_Solanum_lycopersicum_L
[67] Lu L L, Tian S K, Zhang J, et al.Efficient xylem transport and phloem remobilization of Zn in the hyperaccumulator plant species Sedum alfredii[J].New Phytologist, 2013, 198(3):721-731. doi: 10.1111/nph.12168
[68] Kozhevnikova A D, Seregin I V, Erlikh N T, et al. Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens[J].New Phytologist, 2014, 203(2):508-519. doi: 10.1111/nph.12816
[69] Yoneyama T, Ishikawa S, Fujimaki S.Route and regulation of zinc, cadmium, and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling:Metal transporters, metal speciation, grain Cd reduction and Zn and Fe biofortification[J].International Journal of Molecular Sciences, 2015, 16(8):19111-19129. doi: 10.3390/ijms160819111
[70] Gao J, Sun L, Yang X E, et al.Transcriptomic analysis of cadmium stress response in the heavy metal hyperaccumulator Sedum alfredii Hance[J].Plos One, 2013, 8(6):e64643. doi: 10.1371/journal.pone.0064643
[71] Palusińska M, Barabasz A, KozakK, et al.Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes[J].BMC Plant Biology, 2020, 20(1):37. http://www.researchgate.net/publication/338758611_ZnCd_status-dependent_accumulation_of_Zn_and_Cd_in_root_parts_in_tobacco_is_accompanied_by_specific_expression_of_ZIP_genes
[72] Morel M, Crouzet J, Gravot A, et al.AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis[J].Plant Physiology, 2009, 149(2):894-904. doi: 10.1104/pp.108.130294
[73] Ueno D, Yamaji N, Kono I, et al.Gene limiting cadmium accumulation in rice[J].Proceedings of the National Academy of Sciences, 2010, 107(38):16500-16505. doi: 10.1073/pnas.1005396107
[74] Kutrowska A, Szelag M.Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals[J].Acta Physiologiae Plantarum, 2014, 36(8):1957-1968. doi: 10.1007/s11738-014-1576-y
[75] Deng T H B, Tang Y T, Ent A V D, et al.Nickel translocation via the phloem in the hyperaccumulator Noccaea caerulescens (Brassicaceae)[J].Plant and Soil, 2016, 404(1):35-45. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=85119aa52a5bedc95fdc83ed187e48c4
[76] Dan Y B, Zhang W L, Xue R M, et al.Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma-mass spectrometry analysis[J].Environmental Science & Technology, 2017, 49(5):3007-3014. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=809dad9164c921979f01c071084ecd61
[77] Feichtmeier N S, Walther P, Leopold K.Uptake, effects, and regeneration of barley plants exposed to gold nanoparticles[J].Environmental Science & Pollution Research International, 2015, 22(11):8549-8558. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4357b695661430297a547e179ff456e7
[78] Tao Q, Jupa R, Liu Y K, et al.Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii[J].Plant Cell And Environment, 2019, 42(5):1425-1440. doi: 10.1111/pce.13506
[79] Li T Q, Tao Q, Shohag M J I, et al.Root cell wall polysaccharides are involved in cadmium hyperaccumulation in Sedum alfredii[J].Plant and Soil, 2015, 389:387-399. doi: 10.1007/s11104-014-2367-3
[80] Chi K Y, Zou R, Wang L, et al.Cellular distribution of cadmium in two amaranth (Amaranthus mangostanus L.) cultivars differing in cadmium accumulation[J].Environmental Science and Pollution Research, 2019, 26:22147-22158. doi: 10.1007/s11356-019-05390-w
[81] Mori S, Uraguchi S, Ishikawa S, et al.Xylem loading process is a critical factor in determining Cd accumulation in the shoots of Solanum melongena and Solanum torvum[J].Environmental and Experimental Botany, 2009, 67:127-132. doi: 10.1016/j.envexpbot.2009.05.006
[82] Hu P J, Wang Y D, Przybyłowicz W J, et al.Elemental distribution by cryo-micro-PIXE in the zinc and cadmium hyperaccumulator Sedum plumbizincicola grown naturally[J].Plant and Soil, 2015, 388:267-282. doi: 10.1007/s11104-014-2321-4
[83] Wang W J, Zhang M Z, Liu J.Subcellular distribution and chemical forms of Cd in Bougainvillea spectabilis Willd. as an ornamental phytostabilizer:An integrated consideration[J].International Journal of Phytoremediation, 2018, 20(11):1087-1095. doi: 10.1080/15226514.2017.1365335
[84] Rozas M M, Madejon E, Madejon P.Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species:An assessment in sand and soil conditions under different levels of contamination[J].Environmental Pollution, 2016, 216:273-281. doi: 10.1016/j.envpol.2016.05.080
[85] Hou X L, Han H, Cai L P, et al.Pb stress effects on leaf chlorophyll fluorescence, antioxidative enzyme activities, and organic acid contents of Pogonatherum crinitum seedlings[J].Flora, 2018, 240:82-88. doi: 10.1016/j.flora.2018.01.006
[86] Sghaier O M, Merono R M, Zapico E F, et al.Synthesis of a new Cd(Ⅱ)-Ni(Ⅱ) hetero-metallic coordination polymer base on citric acid ligand.X-ray structure, thermal stability, XPS and fluorescence studies[J].Journal of Molecular Structure, 2016, 1105:105-111. doi: 10.1016/j.molstruc.2015.10.028
[87] Zhang Y L, He S R, Zhang Z, et al.Glycine transforma-tioninduces repartition of cadmium and lead in soil constituents[J].Environmental Pollution, 2019, 251:930-937. doi: 10.1016/j.envpol.2019.04.099
[88] Chen S, Lin R Y, Lu H L, et al.Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in kandelia obovata under cadmium and zinc stress[J].Chemosphere, 2020, 249:126341. doi: 10.1016/j.chemosphere.2020.126341
[89] Xin J P, Zhang Y, Tian R N.Tolerance mechanism of Triarrhena saccharifiora (Maxim.) Nakai.seedlings to lead and cadmium:Translocation, subcellular distribution, chemical forms and variations in leaf ultrastructure[J].Ecotoxicology and Environmental Safety, 2018, 165:611-621. doi: 10.1016/j.ecoenv.2018.09.022
[90] Adediran G A, Ngwenya B T, Mosselmans J F W, et al.Mechanisms behind bacteria induced plant growth promotion and Zn accumulation in Brassica juncea[J].Journal of Hazardous Materials, 2015, 283:490-499. doi: 10.1016/j.jhazmat.2014.09.064
[91] Sun J L, Luo L Q.Subcellular distribution and chemical forms of Pb in corn:strategies underlying tolerance in Pb stress[J].Journal of Agricultural and Food Chemistry, 2018, 66(26):6675-6682. doi: 10.1021/acs.jafc.7b03605
[92] Ameen N Z, Amjad M, Murtaza B, et al.Biogeochemical behavior of nickel under different abiotic stresses:Toxicity and detoxification mechanisms in plants[J].Environmental Science and Pollution Research, 2019, 26(11):10496-10514. doi: 10.1007/s11356-019-04540-4
[93] Sunitha M S, Prashant S, Kumar S A, et al.Cellular and molecular mechanisms of heavy metal tolerance in plants:A brief overview of transgenic plants over expressing phytochelatins synthase and metallothionein genes[J].Plant Cell Biotechnology and Molecular Biology, 2013, 13(3):99-104.
[94] Helaoui S, Boughattas I, Hattab S, et al.Physiological, biochemical and transcriptomic responses of Medicago sativa to nickel exposure[J].Chemosphere, 2020, 249:126121. doi: 10.1016/j.chemosphere.2020.126121
[95] Chaudhary K, Agarwal S, Khan S.Role of phytochelatins (PCs), metallothioneins (MTs), and heavy metal atpase (HMA) genes in heavy metal tolerance[M]//Prasad R.Mycoremediation and environmental sustainability: Volume 2.Cham: Springer International Publishing, 2018: 39-60.
[96] Navarrete A, González A, Gómez M, et al.Copper excess detoxification is mediated by a coordinated and complementary induction of glutathione, phytochelatins and metallothioneins in the green seaweed Ulva compressa[J].Plant Physiology and Biochemistry, 2019, 135:423-431. doi: 10.1016/j.plaphy.2018.11.019
[97] Cao Z Z, Qin M L, Lin X Y, et al.Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration[J].Environmental Pollution, 2018, 238:76-84. doi: 10.1016/j.envpol.2018.02.083
[98] Bhargava A, Carmona F F, Bhargava M, et al.Appro-aches for enhanced phytoextraction of heavy metals[J].Journal of Environmental Management, 2012, 105:103-120. http://www.cabdirect.org/abstracts/20123223844.html
[99] Sharma S S, Dietz K J, Mimura T.Vacuolar compart-mentalization as indispensable component of heavy metal detoxification in plants[J].Plant, Cell & Environment, 2016, 39:1112-1126. http://www.ncbi.nlm.nih.gov/pubmed/26729300
[100] Liu H, Zhao H X, Wu L H, et al.Heavy metal ATPase 3(HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola[J].New Phytologist, 2017, 215(2):687-698. doi: 10.1111/nph.14622
[101] Song W Y, Park J, Cózat D G M, et al.Arsenic tolerance in arabidopsis is mediated by two ABCC-type phytochelatin transporters[J].Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(49):21187-21192. doi: 10.1073/pnas.1013964107
[102] Park J, Song W Y, Ko D, et al.The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury[J].The Plant Journal, 2012, 69(2):278-288. doi: 10.1111/j.1365-313X.2011.04789.x
[103] Zhang W W, Hu Y J, Cao Y R, et al.Tolerance of lead by the fruiting body of Oudemansiella radicata[J].Chemosphere, 2012, 88(4):467-475. doi: 10.1016/j.chemosphere.2012.02.079
[104] Srivastava R K, Pandey P, Rajpoot R, et al.Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings[J].Protoplasma, 2014, 251(5):1047-1065. doi: 10.1007/s00709-014-0614-3
[105] Tang K, Zhan J C, Yang H R, et al.Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings[J].Journal of Plant Physiology, 2010, 167(2):95-102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e146b78a8d1cf5b81c94f5cc1e187e9c
[106] Xu X H, Yang B S, Qin G H, et al.Growth, accumulation, and antioxidative responses of two Salix genotypes exposed to cadmium and lead in hydroponic culture[J].Environmental Science and Pollution Research, 2019, 26:19770-19784. doi: 10.1007/s11356-019-05331-7
[107] Shahid M, Dumat C, Pourrut B, et al.Influence of EDTA and citric acid on lead-induced oxidative stress to Vicia faba roots[J].Journal of Soils and Sediments, 2014, 14:835-843. doi: 10.1007/s11368-013-0724-0
[108] Cherif J, Mediouni C, Ammar W B, et al.Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solarium lycopersicum)[J].Journal of Environmental Sciences, 2011, 23(5):837-844. doi: 10.1016/S1001-0742(10)60415-9
[109] Yan Y Y, Yang B, Lan X Y, et al.Cadmium accumulation capacity and resistance strategies of a cadmium-hypertolerant fern-Microsorum fortunei[J].Science of the Total Environment, 2019, 649:1209-1223. doi: 10.1016/j.scitotenv.2018.08.281
[110] 黑泽文, 向慧敏, 章家恩, 等.水合欢对重金属Cd、Pb的耐受性及吸收富集特性[J].生态毒理学报, 2019, 14(3):286-296. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cyyhj201903029
Hei Z W, Xiang H M, Zhang Z E, et al.Tolerance and accumulation ability of Neptunia olerace to Cd and Pb stress in soil[J].Asian Journal of Ecotoxicology, 2019, 14(3):286-296. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cyyhj201903029
[111] Guo H, Jiang J W, Gao J Q, et al.Evaluation of cadmium hyperaccumulation and tolerance potential of Myriophyllum aquaticum[J].Ecotoxicology and Environmental Safety, 2020, 195:110502. doi: 10.1016/j.ecoenv.2020.110502
[112] Małecka A, Konkolewska A, Han A, et al.Insight into the phytoremediation capability of Brassica juncea (v.Malopolska):Metal accumulation and antioxidant enzyme activity[J].International Journal of Molecular Sciences, 2019, 20:4355. doi: 10.3390/ijms20184355
[113] Chandrasekhara C, Ray J G.Lead accumulation, growth responses and biochemical changes of three plant species exposed to soil amended with different concentrations of lead nitrate[J].Ecotoxicology and Environmental Safety, 2019, 171:26-36. doi: 10.1016/j.ecoenv.2018.12.058
[114] Zhang C L, Chen Y Q, Xu W H, et al.Resistance ofAlfalfa and Indian mustard to Cd and the correlation of plant Cd uptake and soil Cd form[J].Environmental Science and Pollution Research, 2019, 26:13804-13811. doi: 10.1007/s11356-018-3162-0
[115] Reeves R D, Baker A J M, Tanguy Jaffre, et al.A global database for plants that hyperaccumulate metal and metalloid trace elements[J].New Phytologist, 2018, 218:407-411. doi: 10.1111/nph.14907
[116] Rosenkranz T, Hipfinger C, Ridard C, et al.A nickel phytomining field trial using Odontarrhena chalcidica and Noccaea goesingensis on an Austrian serpentine soil[J].Journal of Environmental Management, 2019, 242:522-528. doi: 10.1016/j.jenvman.2019.04.073
[117] Siebert S J, Schutte N C, Bester S P, et al.Senecio conrathii N.E.Br.(Asteraceae), a new hyperaccumulator of nickel from serpentinite outcrops of the Barberton Greenstone Belt, South Africa[J].Ecological Research, 2018, 33:651-658. doi: 10.1007/s11284-017-1541-5
[118] Yamaguchi T, Tomioka R, Takenaka C.Accumulation of cobalt and nickel in tissues of Clethra barbinervis in a metal dosing trial[J].Plant and Soil, 2017, 421:273-283. doi: 10.1007/s11104-017-3455-y
[119] Shu H Y, Zhang J, Liu F Y, et al.Comparative transcriptomic studies on a cadmium hyperaccumulator Viola baoshanensis and its non-tolerant counterpart V.inconspicua[J].International Journal of Molecular Sciences, 2019, 20:1906. doi: 10.3390/ijms20081906
[120] Potdukhe R M, Bedi P, Sarangi B K, et al.Root transcripts associated with arsenic accumulation in hyperaccumulator Pteris vittata[J].Journal of Biosciences, 2018, 43(1):105-115. doi: 10.1007/s12038-018-9735-8
[121] 张云霞, 宋波, 宾娟, 等.超富集植物藿香蓟(Ageratum conyzoides L.)对镉污染农田的修复潜力[J].环境科学, 2019, 40(5):2453-2459. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201905055
Zhang Y X, Song B, Bin J, et al.Remediation potential of Ageratum conyzoides L.on cadmium contaminated farmland[J].Environmental Science, 2019, 40(5):2453-2459. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201905055
[122] Willscher S, Jablonski L, Fona Z, et al.Phytoremediation experiments with Helianthus tuberosus under different pH and heavy metal soil concentrations[J].Hydrometallurgy, 2017, 168:153-158. doi: 10.1016/j.hydromet.2016.10.016
[123] Kabas S, Mella F S, Huynh T, et al.Metal uptake and organic acid exudation of native Acacia species in mine tailings[J].Australian Journal of Botany, 2017, 65(4):357-367. doi: 10.1071/BT16189
[124] Wang L W, Hou D Y, Shen Z T, et al.Field trials of phytomining and phytoremediation:A critical review of influencing factors and effects of additives[J].Critical Reviews in Environmental Science and Technology, 2019, 51. http://www.tandfonline.com/doi/full/10.1080/10643389.2019.1705724
[125] Bhargava A, Fuentes F, Shukla S, et al.Genetic variability in vegetable Chenopodium for morphological and quality traits over different cuttings[J].Ciencia e Investigacion Agraria, 2019, 46(2):179-186. doi: 10.7764/rcia.v46i2.2145
[126] Ruiz O N, Daniell H.Genetic engineering to enhance mercury phytoremediation[J].Current Opinion in Biotechnology, 2009, 20:213-219. doi: 10.1016/j.copbio.2009.02.010
[127] Valdez E G, Alarcóna A, Cerrato R F, et al.Induced accumulation of Au, Ag and Cu in Brassica napus grown in a mine tailings with the inoculation of Aspergillus niger and the application of two chemical compounds[J].Ecotoxicology and Environmental Safety, 2018, 154:180-186. doi: 10.1016/j.ecoenv.2018.02.055
[128] Harris A T, Naidoo K, Nokes J, et al.Indicative assessment of the feasibility of Ni and Au phytomining in Australia[J].Journal of Cleaner Production, 2009, 17(2):194-200. doi: 10.1016/j.jclepro.2008.04.011
[129] 刘婷婷, 彭程, 王梦, 等.海州香薷根细胞壁对铜的吸附固定机制研究[J].环境科学学报, 2014, 34(2):514-523. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkxxb201402033
Liu T T, Peng C, Wang M, et al.Mechanism of fixation and adsorption of copper on root cell wall of Elsholtzia splendens[J].Acta Scientiae Circumstantiae, 2014, 34(2):514-523. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkxxb201402033
[130] 何宇宁, 徐仲瑞, 熊治廷.铜胁迫对不同抗性种群海州香薷酸性转化酶基因启动子甲基化的影响[J].植物科学学报, 2017, 35(4):574-582. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=whzwxyj201704013
He Y N, Xu Z R, Xiong Z T.DNA methylation patterns of acid invertase gene promoters from Cu-tolerant and non-tolerant populations of Elsholtzia haichowensis under copper stress[J].Plant Science Journal, 2017, 35(4):574-582. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=whzwxyj201704013
[131] Farjandi F, Faiziev A, Fozilov M, et al.The application of biogeochemistry for gold exploration in the Masjed-Daghi, Julfa, NW Iran[J].Arabian Journal of Geosciences, 2013, 6(5):1435-1446. doi: 10.1007/s12517-011-0448-7
-



 下载:
下载: