Effect of Mechanical Force on Activation Characteristics of a Low-grade Ascharite Ore
-
摘要:
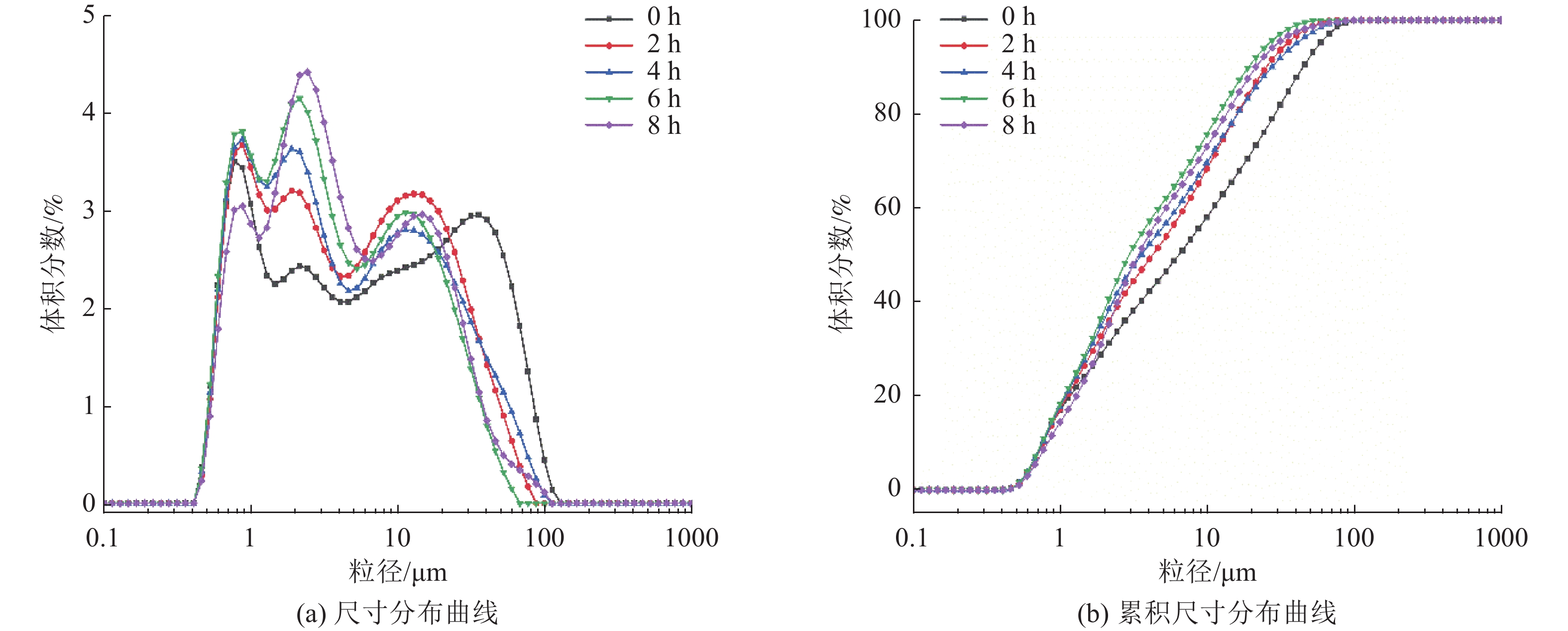
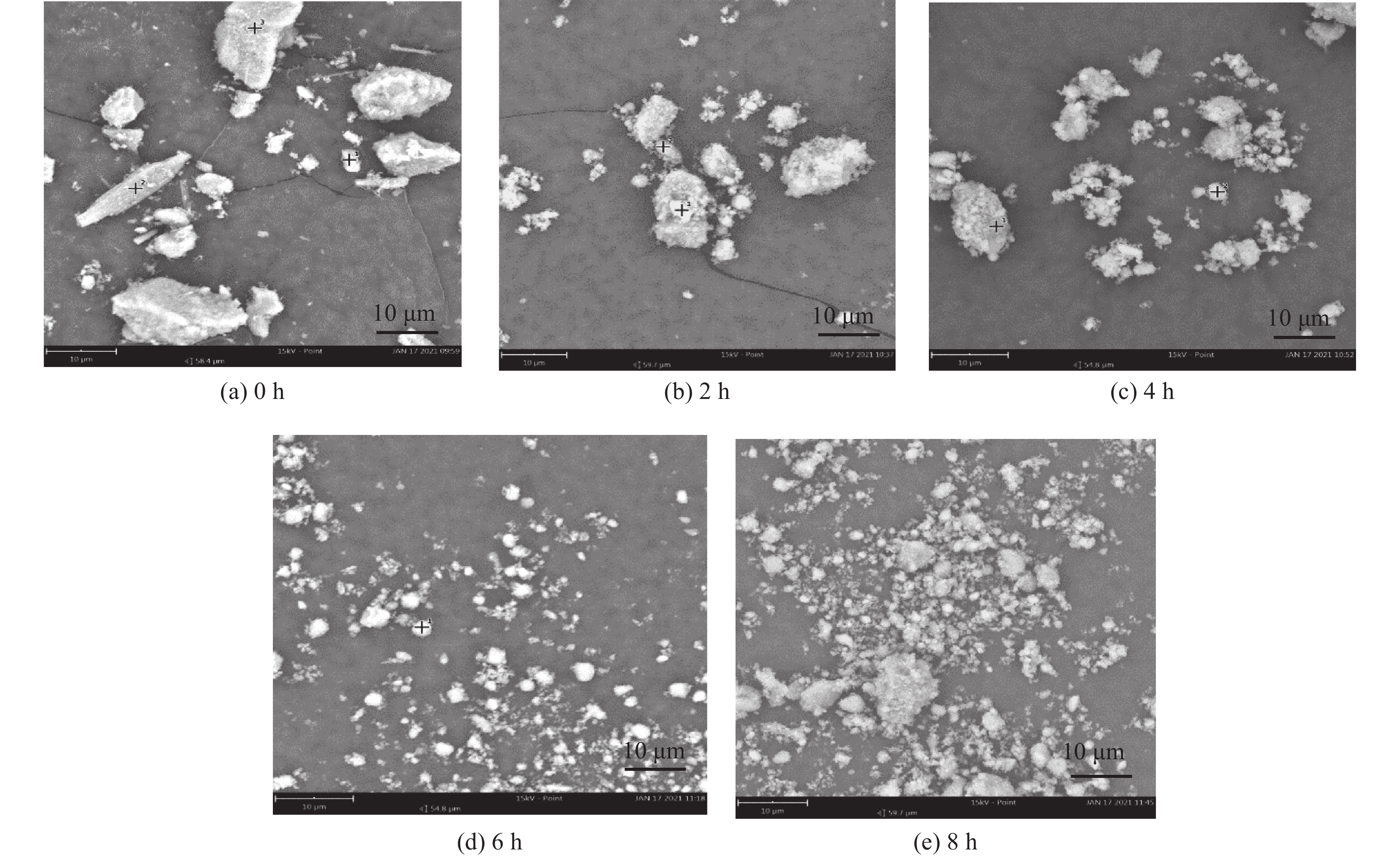
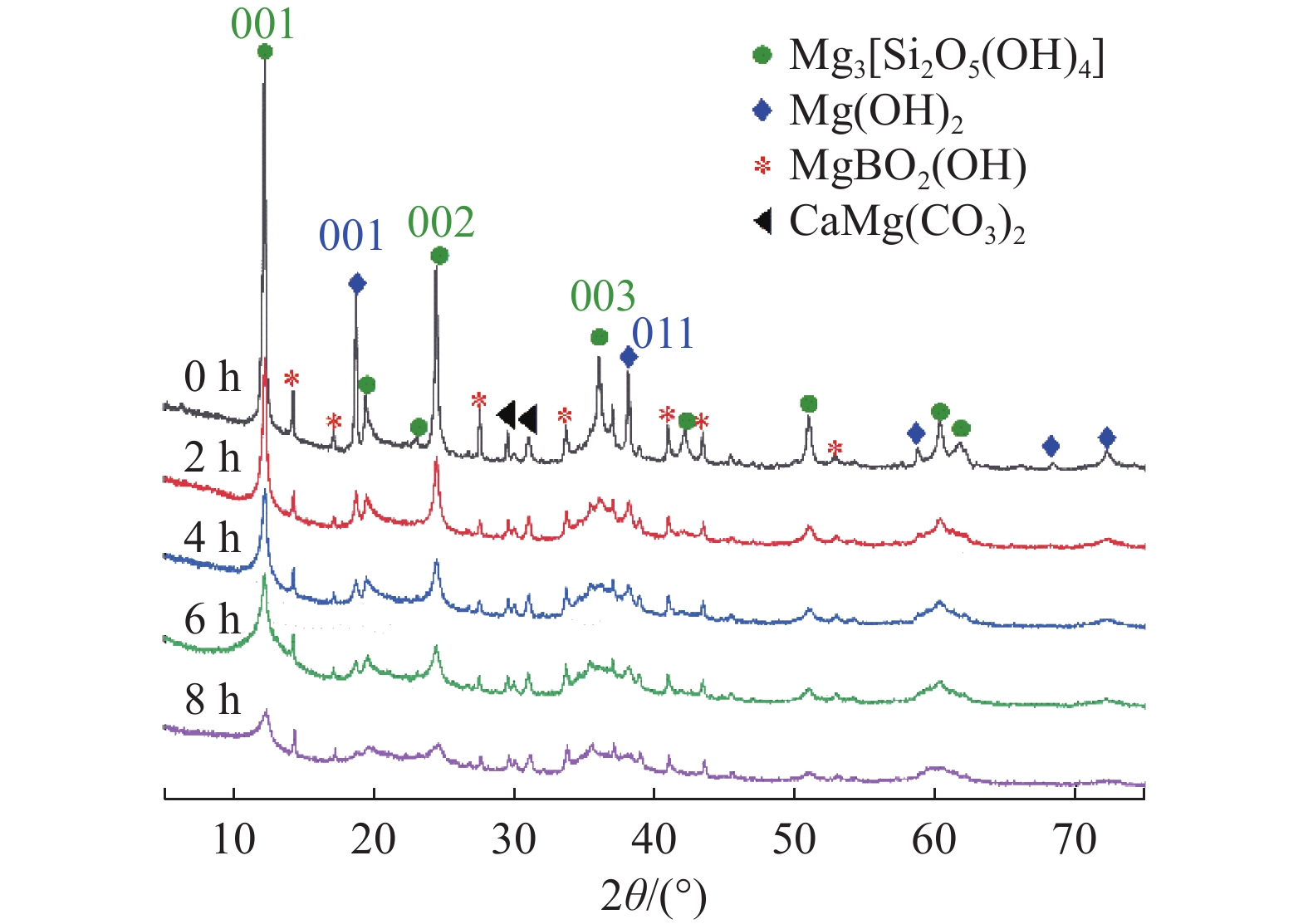
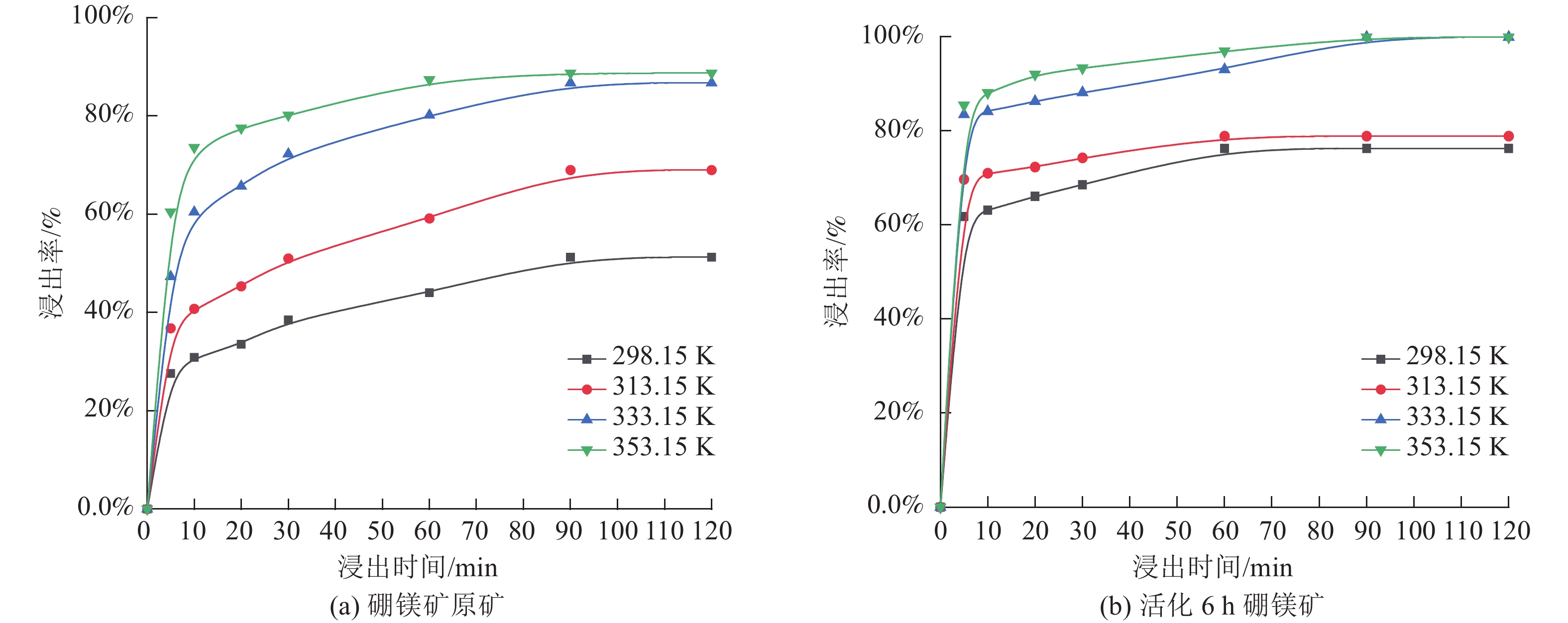
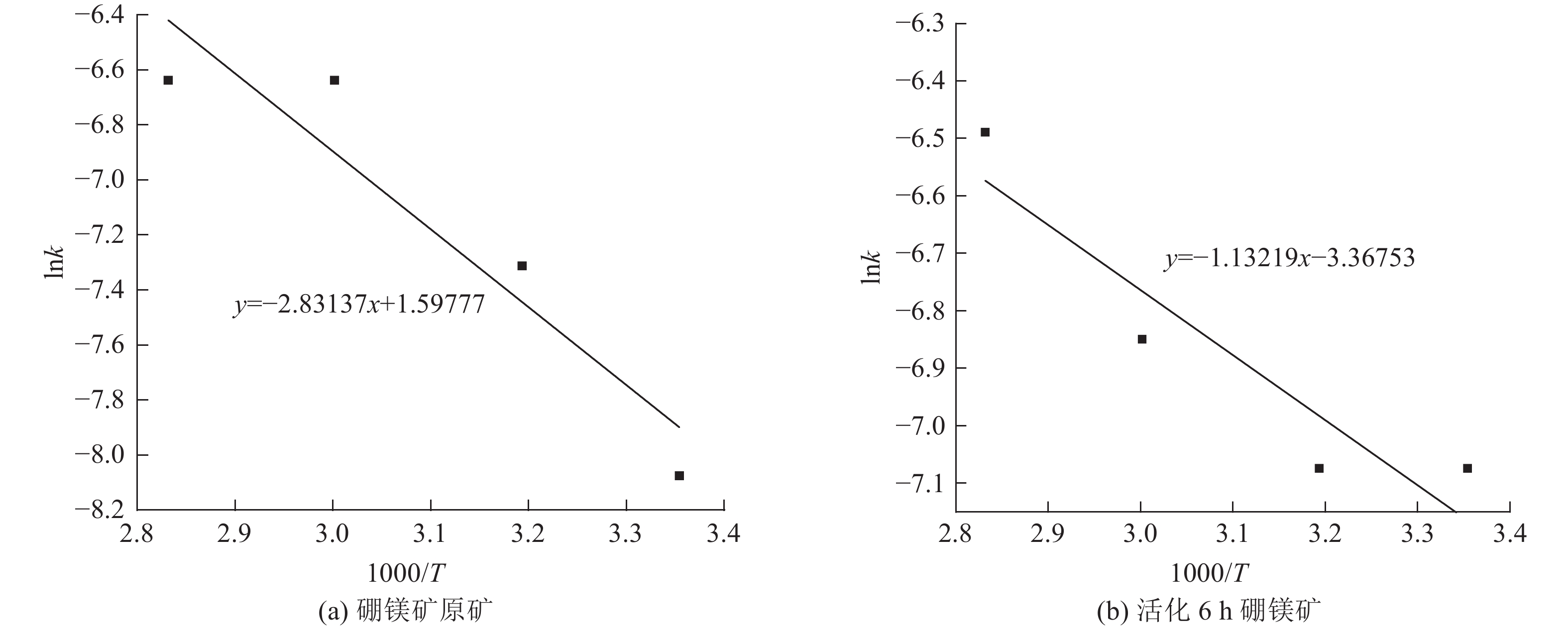
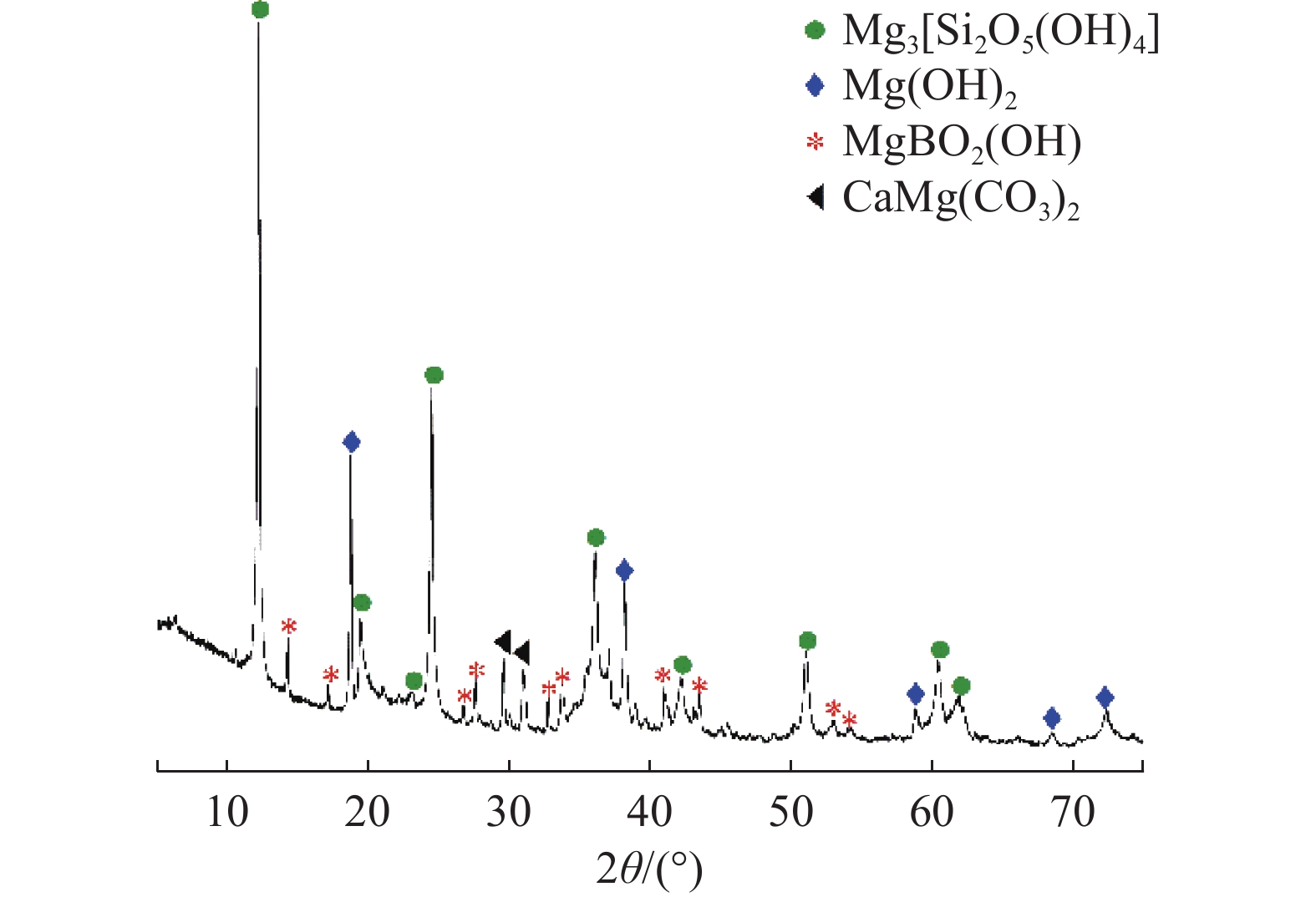
这是一篇矿业工程领域的论文。本文通过SEM-EDS、XRD、FT-IR、激光粒度分析等手段对机械活化后的硼镁矿粉进行了表征。结果表明硼镁矿的晶体结构在机械力的作用下发生改变,由规则有序的晶态结构向无序的非晶态结构转变。矿粉粒度分布受机械力的影响作用较大,在球磨机转速200 r/min和球料比10∶1的实验条件下,较佳活化时间为6 h,此时90%的矿粉粒径小于21.5 μm;硼镁矿活性与活化后的活性键的数量有关,在一定的活化时间内,硼镁矿活性随着活化时间的增加而提高,但活化时间过长矿粉颗粒在范德华力的作用下发生团聚,反而导致活性降低;机械活化预处理能够降低硼镁矿浸出反应中对硫酸浓度和温度的依赖,显著提高硼镁矿中元素浸出率,实验表明在反应温度25 ℃、硫酸浓度1.48 mol/L条件下,活化6 h的硼镁矿浸出率较硼镁矿原矿提高了50%,且在较为友好的条件下可制备得到非晶态SiO2。经活化后的硼镁矿与硫酸反应的表观活化能由23.54 kJ/mol减小为9.41 kJ/mol,浸出过程中固体产物层的内扩散是影响硼镁矿与硫酸反应的限制性环节。
Abstract:This is an article in the field of mining engineering. In this article, SEM-EDS, XRD, FT-IR, laser particle size analysis and other means were used to characterize the mechanical activation of boron and magnesium ore powder. The results show that the crystal structure of boraxite changes at the action of mechanical force from the regular and orderly crystal structure to the disorder of the amorphous structure. The particle size distribution of the ore powder is greatly affected by the mechanical force. At the experimental conditions of 200 r/min speed of the ball mill and the ratio of ball to material of 10∶1, the optimal activation time is 6 h, when the particle size of 90% of the ore powder is less than 21.5 μm. The activity of ascharite is related to the number of active bonds after activation. In a certain activation time, the activity of ascharite increases with increasing activation time, but the agglomeration of mineral powder particles at the action of van der Waals force for a long activation time leads to a decrease in activity. The mechanical activation pretreatment can reduce the dependence on sulfuric acid concentration and temperature in the leaching reaction of ascharite, and significantly improve the leaching rate of elements in ascharite. The experiments show that the leaching rate of ascharite after activation for 6 h is 50% higher than that of the raw ascharite under the condition of reaction temperature 25 ℃ and sulfuric acid concentration 1.48 mol/L. And amorphous SiO2 can be prepared at friendly conditions. The apparent activation energy decreases from 23.54 kJ/mol to 9.41 kJ/mol after activation. The diffusion of the solid product layer during the leaching process is the limiting link which affects the reaction of ascharite with sulfuric acid.
-

-
表 1 低品位硼镁矿的主要化学成分/%
Table 1. Main chemical composition of the boron-magnesium ore
B2O3 MgO CaO SiO2 Fe2O3 Al2O3 LOI 9.46 48.7 1.68 28.67 2.11 0.47 8.91 表 2 不同机械活化时间下矿粉粒度的相关数据
Table 2. Correlation data of ore particle size at different mechanical activation time
机械活化时间 D50/μm D90/μm 比表面积/(m2/kg) 0 7.2 50.4 2 270 2 4.78 28.4 2 498 4 4.00 31.0 2 599 6 3.33 21.5 2 756 8 3.82 24.1 2 439 -
[1] 王松, 赵元艺, 汪傲, 等. “一带一路”国家钾盐及硼资源分布规律与开采技术[J]. 地质通报, 2017, 36(1):35-49.WANG S, ZHAO Y Y, WANG A, et al. Distribution pattern of potash and boron resources and mining technology in “Belt and Road” countries[J]. Geological Bulletin, 2017, 36(1):35-49. doi: 10.3969/j.issn.1671-2552.2017.01.004
WANG S, ZHAO Y Y, WANG A, et al. Distribution pattern of potash and boron resources and mining technology in “Belt and Road” countries[J]. Geological Bulletin, 2017, 36(1):35-49. doi: 10.3969/j.issn.1671-2552.2017.01.004
[2] 李杰, 刘艳丽, 刘素兰, 等. 低品位硼镁矿制备硼酸及回收硫酸镁的研究[J]. 矿产综合利用, 2009(1):3-7.LI J, LIU Y L, LIU S L, et al. Preparation of boric acid and recovery of magnesium sulfate from low-grade boron and magnesium ores[J]. Multipurpose Utilization of Mineral Resources, 2009(1):3-7. doi: 10.3969/j.issn.1000-6532.2009.01.001
LI J, LIU Y L, LIU S L, et al. Preparation of boric acid and recovery of magnesium sulfate from low-grade boron and magnesium ores[J]. Multipurpose Utilization of Mineral Resources, 2009(1):3-7. doi: 10.3969/j.issn.1000-6532.2009.01.001
[3] 乌图那顺, 仲剑初, 何丹, 等. 高镁低品位硼镁矿的综合利用[J]. 化工矿物与加工, 2010, 39(5):8-11.UTU N S, ZHONG J C, HE D, et al. Comprehensive utilization of high-magnesium low-grade boromagnesite[J]. Chemical Minerals and Processing, 2010, 39(5):8-11. doi: 10.3969/j.issn.1008-7524.2010.05.003
UTU N S, ZHONG J C, HE D, et al. Comprehensive utilization of high-magnesium low-grade boromagnesite[J]. Chemical Minerals and Processing, 2010, 39(5):8-11. doi: 10.3969/j.issn.1008-7524.2010.05.003
[4] 刘璇, 李如燕, 崔孝炜, 等. 机械力对高硅钒尾矿活化性能的影响 [J]. 硅酸盐通报, 2019, 38(8): 2662-2668.LIU X , LI R Y, CUI X W, et al. Influence of mechanical force on activation properties of high silica vanadium tailings [J]. Silicate Bulletin, 2019, 38(8): 2662-7.
LIU X , LI R Y, CUI X W, et al. Influence of mechanical force on activation properties of high silica vanadium tailings [J]. Silicate Bulletin, 2019, 38(8): 2662-7.
[5] 李冷, 曾宪滨. 粉碎机械力化学的进展及其在材料开发中的应用 [J]. 武汉工业大学学报, 1993(1): 23-28.LI L, ZENG X B. Advances in pulverizing mechanical force chemistry and its application to materials development [J]. Journal of Wuhan University of Technology, 1993(1): 23-6.
LI L, ZENG X B. Advances in pulverizing mechanical force chemistry and its application to materials development [J]. Journal of Wuhan University of Technology, 1993(1): 23-6.
[6] FROST R L, SCHOLZ R, LóPEZ A, et al. The molecular structure of theborate mineral szaibelyite MgBO2(OH) – A vibrational spectroscopic study [J]. Journal of Molecular Structure, 2015, 1089(20-4.
[7] 冯其明, 杨艳霞, 刘琨, 等. 纤蛇纹石纳米纤维的制备及表征[J]. 中南大学学报(自然科学版), 2006(6):1081-1086.FENG Q M, YANG Y X, LIU K, et al. Preparation and characterization of fibrous serpentine nanofibers[J]. Journal of Central South University (Natural Science Edition), 2006(6):1081-1086.
FENG Q M, YANG Y X, LIU K, et al. Preparation and characterization of fibrous serpentine nanofibers[J]. Journal of Central South University (Natural Science Edition), 2006(6):1081-1086.
[8] 李幼琴, 江绍英, 范文伟. 蛇纹石族矿物的红外光谱特征及其研究[J]. 地质科学, 1981(3):247-53.LI Y Q, JIANG S Y, FAN W W. Infrared spectral characteristics of serpentine minerals and their study[J]. Geoscience, 1981(3):247-53.
LI Y Q, JIANG S Y, FAN W W. Infrared spectral characteristics of serpentine minerals and their study[J]. Geoscience, 1981(3):247-53.
[9] 杨艳霞, 冯其明, 刘琨, 等. 纤蛇纹石的盐酸浸出及其动力学模型研究[J]. 材料导报, 2007(3):136-145.YANG Y X, FENG Q M, LIU K, et al. Hydrochloric acid leaching of fibrous serpentine and its kinetic modeling[J]. Materials Letters, 2007(3):136-145. doi: 10.3321/j.issn:1005-023X.2007.03.037
YANG Y X, FENG Q M, LIU K, et al. Hydrochloric acid leaching of fibrous serpentine and its kinetic modeling[J]. Materials Letters, 2007(3):136-145. doi: 10.3321/j.issn:1005-023X.2007.03.037
[10] 聂正林, 宋广翰, 孟雯, 等. 机械活化对废荧光粉中钇浸出动力学的影响[J]. 环境工程学报, 2019, 13(6):1 410-1416.NIE Z L, SONG G H, MENG W, et al. Effect of mechanical activation on the leaching kinetics of yttrium from waste phosphors[J]. Journal of Environmental Engineering, 2019, 13(6):1 410-1 416. doi: 10.12030/j.cjee.201810007
NIE Z L, SONG G H, MENG W, et al. Effect of mechanical activation on the leaching kinetics of yttrium from waste phosphors[J]. Journal of Environmental Engineering, 2019, 13(6):1 410-1 416. doi: 10.12030/j.cjee.201810007
[11] BEOLCHINI F, PAPINI M P, TORO L, et al. Acid leaching of manganiferous ores by sucrose: Kinetic modelling and related statistical analysis[J]. Minerals Engineering, 2001, 14(2):175-84. doi: 10.1016/S0892-6875(00)00173-4
[12] LIDDELL K C. Shrinking core models in hydrometallurgy: What students are not being told about the pseudo-steady approximation[J]. Hydrometallurgy, 2005, 79(1):62-68.
[13] 赵青. 碱式硫酸铬清洁制备工艺的基础研究 [D]. 沈阳: 东北大学, 2015.ZHAO Q. Fundamental research on clean preparation process of basic chromium sulfate [D]. Shenyang: Northeastern University, 2015.
ZHAO Q. Fundamental research on clean preparation process of basic chromium sulfate [D]. Shenyang: Northeastern University, 2015.
[14] 王玲, 崔兆纯, 李存国, 等. 橄榄石常压硫酸溶解过程中镁的浸出动力学研究[J]. 矿产综合利用, 2020(5):186-190.WANG L, CUI Z C, LI C G, et al. Kinetics of magnesium leaching from olivine during sulfuric acid dissolution at atmospheric pressure[J]. Multipurpose Utilization of Mineral Resources, 2020(5):186-190. doi: 10.3969/j.issn.1000-6532.2020.05.029
WANG L, CUI Z C, LI C G, et al. Kinetics of magnesium leaching from olivine during sulfuric acid dissolution at atmospheric pressure[J]. Multipurpose Utilization of Mineral Resources, 2020(5):186-190. doi: 10.3969/j.issn.1000-6532.2020.05.029
[15] 张建树, 张荣, 毕继诚. CO2矿化反应基础研究Ⅰ. 镁橄榄石和蛇纹石盐酸浸出动力学研究[J]. 燃料化学学报, 2011, 39(9):706-11.ZHANG J S, ZHANG R, BI J C. Basic research on CO2 mineralization reaction Ⅰ. Kinetics of hydrochloric acid leaching of magnesium olivine and serpentine[J]. Journal of Fuel Chemistry, 2011, 39(9):706-11.
ZHANG J S, ZHANG R, BI J C. Basic research on CO2 mineralization reaction Ⅰ. Kinetics of hydrochloric acid leaching of magnesium olivine and serpentine[J]. Journal of Fuel Chemistry, 2011, 39(9):706-11.
-



 下载:
下载: