Study on Removal of Fluoride and Nitride in Secondary Aluminum Dross by High Temperature Roasting
-
摘要:
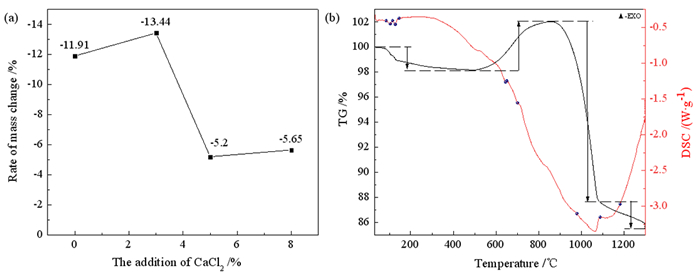
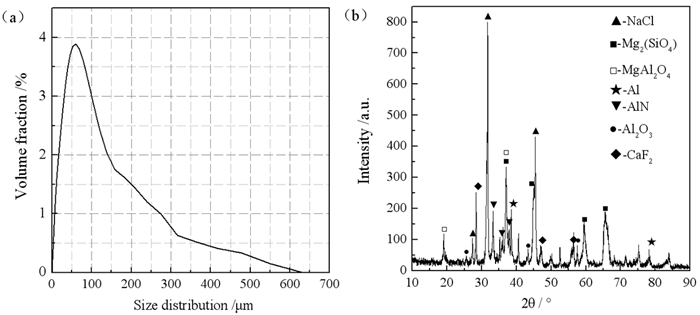
铝灰中的氮化铝受潮产生氨气和部分氟化物可溶是铝灰成为危险固体废弃物的主要原因,为脱除铝灰中的氮化铝和氟化物,实现铝灰的无害化、减量化的目的,采用钙盐高温焙烧铝灰脱氮固氟、水洗回收无机盐的工艺,研究了焙烧温度和加入钙盐的质量对铝灰脱氮固氟效果的影响。结果表明,在加入3%的CaCl2作为固氟剂、焙烧温度为1 300℃和焙烧时间为4 h的条件下的铝灰氮元素含量降低至0%,铝灰遇水气体释放量降低至2 mL/g,可溶出氟离子浓度降低至6.71 mg/L,铝灰减重13.44%,焙烧后的铝灰水洗无机盐的回收率为84%。研究为铝灰的无害化减量化资源化提供了新的思路。
Abstract:Ammonia caused by aluminum nitride being damp and fluoride pollution of groundwater are the main reasons why aluminum dross becomes a hazardous solid waste.In order to remove aluminum nitride and fluoride in aluminum dross, the purpose of harmless reduction of aluminum dross is achieved.Using the process of adding calcium salt for high-temperature roasting and washing recovery, the effects of roasting temperature and the quality of added calcium salt on the denitrification and fluoride fixation of aluminum dross were studied.The results showed that the addition of CaCl2 with a mass fraction of 3% as a fluorine-fixing agent, a roasting temperature of 1 300 ℃, and a roasting time of 4 h, under these conditions, the content of aluminum dross nitrogen decreased to 0%, reduced the aluminum dross gas release to 2 mL/g and soluble fluoride ion concentration to 6.71 mg/L, the weight loss of aluminum dross is 13.44%.The aluminum dross calcined under this condition was washed with water, and the remaining inorganic salt components were recovered with a recovery rate of 84%. The research provides a new idea for the harmless reduction and recycling of aluminum dross.
-
Key words:
- secondary aluminum dross /
- aluminum nitride /
- harmless /
- high-temperature roasting
-

-
表 1 二次铝灰元素及含量分析(XRF)
Table 1. SAD element and content analysis
名称 Al O Mg Na Cl K F V Ti Ca Si Fe 含量/% 33.943 29.476 14.209 9.542 3.449 2.865 2.579 1.142 1.023 0.994 0.696 0.083 -
[1] SAMSON OLUROPO ADEOSUN, OLATUNDE ISRAEL SEKUNOWO, OMOTAYO OLUWASEYI TAIWO, et al. Physical and mechanical properties of aluminum dross[J]. Advances in Materials, 2014, 3(2): 48-51.
[2] ABDRAHIM ABDULKADIR, ADESOLA AJAYI, MOHAMED I HASSAN. Evaluating the chemical composition and the molar heat capacities of a white aluminum dross[J]. Energy Procedia, 2015, 75: 2099-2105. doi: 10.1016/j.egypro.2015.07.326
[3] MAHINROOSTA MOSTAFA, ALLAHVERDI ALI. Enhanced alumina recovery from secondary aluminum dross for high purity nanostructured γ-alumina powder production: kinetic study[J]. J. Environ Manage, 2018, 212: 278-291. doi: 10.1016/j.jenvman.2018.02.009
[4] ARUNABH MESHRAM, KAMALESH KUMAR SINGH. Recovery of valuable products from hazardous aluminum dross: a review[J]. Resources, Conservation & Recycling, 2018, 130: 95-108.
[5] MIRIAN CHIEKOSHINZATO, RAPHAEL HYPOLITO. Effect of disposal of aluminum recycling waste in soil and water bodies[J]. Environmental Earth Sciences, 2016, 75(7).
[6] TAKEHITO HIRAKI, TETSUYA NAGASAKA. An easier upgrading process of aluminum dross residue by screening technique[J]. Journal of Material Cycles and Waste Management, 2015, 17(3): 566-573. doi: 10.1007/s10163-014-0283-5
[7] 邴纯. 铝灰在焙烧中的变化及铝灰返电解槽的试验研究[D]. 沈阳: 东北大学, 2012.
[8] 张宁燕. 碱法焙烧脱除二次铝灰中的氟、氯元素及铝的回收[J]. 新疆有色金属, 2019, 42(2): 47-49. https://www.cnki.com.cn/Article/CJFDTOTAL-XJYS201902024.htm
[9] MOSTAFA MAHINROOSTA, ALI ALLAHVERDI. Hazardous aluminum dross characterization and recycling strategies: a critical review[J]. J. Environ Manage, 2018, 223: 452-468. doi: 10.1016/j.jenvman.2018.06.068
[10] 武正君, 宋良杰. 铝电解过程危险废物的资源化利用技术[J]. 环境科学导刊, 2019, 38(5): 75-78. https://www.cnki.com.cn/Article/CJFDTOTAL-YNHK201905018.htm
[11] 王榕林, 孙加林, 卜景龙, 等. AlN微粉非等温氧化动力学研究[J]. 材料工程, 2011(4): 29-32, 37. https://www.cnki.com.cn/Article/CJFDTOTAL-CLGC201104008.htm
-



 下载:
下载: