Ultrasonic Extraction of Trace Iron in Iron Tailings with Ionic Liquid [Emim]PF6-Phenanthroline
-
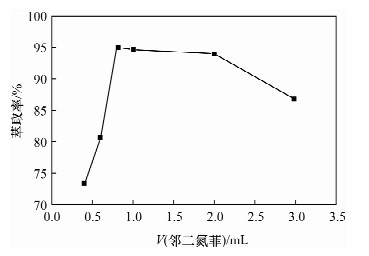
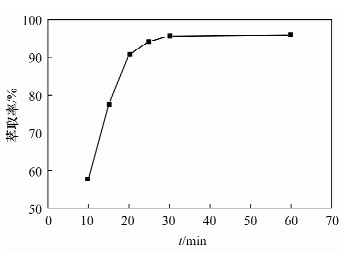
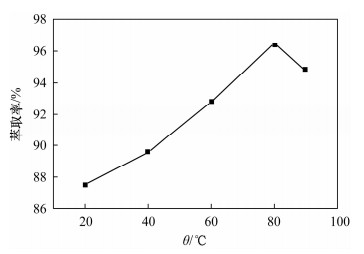
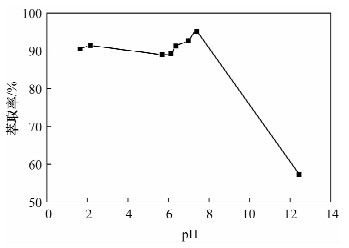
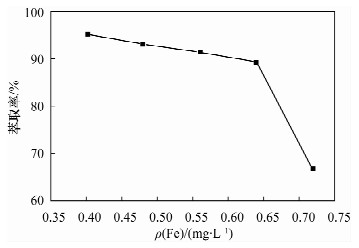
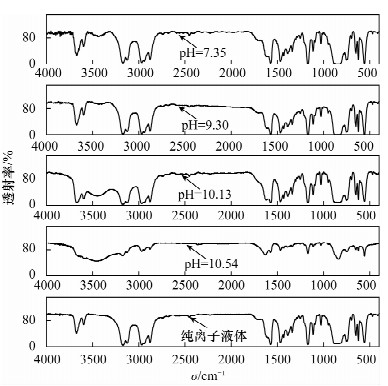
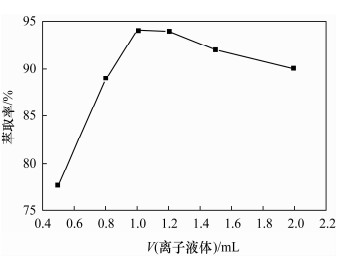
摘要: 铁尾矿不仅造成资源的浪费,同时也给环境带来严重的污染。针对铁尾矿具有组分复杂且铁含量较低的特点,本文采用离子液体[Emim]PF6-邻二氮菲超声萃取分离体系,对铁尾矿样品中的铁进行萃取。为提高铁尾矿中铁的萃取率,考察了离子液体用量、温度、pH值等萃取条件对萃取率的影响。结果表明,在萃取体系中加入0.8 mL邻二氮菲、1.0 mL离子液,控制温度80℃、pH值为7.3、萃取时间为30 min时,铁矿泥中微量铁的萃取率可达92.74%,而且共存离子K+、Na+、Ca2+、Mg2+、Zn2+不影响水相中铁的测定。对使用后的离子液体进行反萃取研究发现,当反萃取时的pH > 11时,回收的离子液体稳定性降低;而低温加热对使用后的离子液体反萃取影响较小,使用后的离子液体经NaOH处理回收,其红外谱图与使用前基本一致,表明回收的离子液体可重复使用。与传统的液液萃取方法相比,本萃取方法具有离子液体用量少、萃取率高、易回收等特点,可用于铁尾矿中金属铁的回收利用。Abstract: Iron tailings are not only a waste of resource, but also bring serious environmental pollution. On account of the complex components and lower iron content in iron tailings, the ultrasonic extraction system of ionic liquid [Emim]PF6-phenanthroline to extract trace iron from iron mud samples has been improved and the method is described in this paper. In order to improve the iron extraction rate from iron tailings, the effect factors were studied, such as the ionic liquid dosage, temperature and pH value on the extraction rate. The results show that the extraction rate of the trace iron from iron mud reached 92.74% under the addition of 0.8 mL phenanthroline, 1.0 mL ionic liquid, and the pH value was 7.3 when the extraction time was 30 minutes at 80℃. Moreover, the co-existence ions of K+, Na+, Ca2+, Mg2+ and Zn2+ had no effect on iron determination. Meanwhile, the back extraction study for the used ionic liquid revealed that the stability of the recovered ionic liquid was lowered when the pH value was more than 11. However, low temperature heating had relatively slight effects for back extraction on the used ionic liquid. The used ionic liquid can be treated and recovered with NaOH solution and the infrared spectrogram remained almost the same as the new ones, which can be reused after the recovery. Compared with the traditional methods of liquid-liquid extraction, this method has the advantages of lower dosage of ionic liquid, higher rate of extraction and easier recovery, and it can be applied to the recovery of the iron from iron tailings.
-
Key words:
- iron tailing /
- iron /
- ionic liquid /
- phenanthroline /
- ultrasonic extraction
-

-
表 1 样品分析结果
Table 1. Analytical results of actual sample
ρ(Fe)/(μg·mL-1) 回收率/% RSD/%
(n=5)w(Fe)/% 本法
测定值加标量 加标后
测定值ICP-AES
法测定值3.743 1.805 5.508 3.804 97.78 6.2 6.5 3.743 3.697 7.392 3.803 98.70 3.743 7.480 11.140 3.796 98.89 -
[1] 张淑会,薛向欣,金在峰.我国铁尾矿的资源现状及其综合利用[J].材料与冶金学报,2004,4(3): 241-245. http://www.cnki.com.cn/Article/CJFDTOTAL-HUJI200404001.htm
[2] 张渊,李俊峰,索崇慧.矿山尾矿综合利用及其环境治理的意义[J].农业技术,2000,20(4): 56-57. http://www.cnki.com.cn/Article/CJFDTOTAL-NYYS200004022.htm
[3] Das S K, Kumar S, Ramachandraro P.Exploitation of iron orefor the development of ceramic tiles [J].Waste Management,2000,20: 725-729. doi: 10.1016/S0956-053X(00)00034-9
[4] Licskó I, Lois L, Szebényi G.Tailings as a source of environmental pollution[J].Water Science Technology,1999,39(10-11): 333-336
[5] Matschullat J, Borba R P, Deschamps E.Human and environmental contamination in the iron quadrangle [J].Applied Geochemistry,2000,15: 193-201.
[6] 秦煜民.磁选尾矿铁资源回收利用现状与前景[J].中国矿业,2010,19(5): 47-49. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGKA201005018.htm
[7] 牟联胜.云南某铁选厂尾矿回收赤褐铁矿的生产实残[J].现代矿业,2010,27(1): 105-106. http://www.cnki.com.cn/Article/CJFDTOTAL-KYKB201001045.htm
[8] 曾茂青,王世涛,杨晓峰,乐智广,孙广周.铁尾矿二次资源的综合利用[J].矿冶,2011,20(4): 57-59. http://www.cnki.com.cn/Article/CJFDTOTAL-KYZZ201104014.htm
[9] 郭立颖,史铁钧,李忠,段衍朋.两种咪唑类离子液体对杉木粉溶解性能的研究[J].化工学报,2008,59(5): 1299-1304.
[10] 姜大雨,朱红,王良.离子液体双水相萃取的应用研究进展[J].化学试剂,2010,32(9): 805-810. http://www.cnki.com.cn/Article/CJFDTOTAL-HXSJ201009012.htm
[11] Swatloski R P, Spear S K, Holbrey J D, Rogers R D. Dissolution of cellulose with ionic liquids [J].Journal of the American Chemical Society,2002,124(18): 4974-4795. doi: 10.1021/ja025790m
[12] Zhang H, Wu J, Zhang J, He J S.1-allyl-3-methylimi-dazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose[J].Macromolecules,2005,38(20): 8272-8277. doi: 10.1021/ma0505676
[13] 高微,于泓,周爽.色谱分析中离子液体的应用及其测定[J].色谱,2010,28(1): 14-22. http://www.cnki.com.cn/Article/CJFDTOTAL-WGTX201604115.htm
[14] 孙晓琦,徐爱梅,陈继,李德谦.离子液基萃取金属离子的研究进展[J].分析化学,2007,35(4): 597-604. http://www.cnki.com.cn/Article/CJFDTOTAL-FXHX200704035.htm
[15] 李长平,辛宝平.疏水性离子液体为溶剂对Co2+和Cd2+废水的萃取性能[J].过程工程学报,2007,7(4): 674-678.
[16] 陈莉莉,邱祖民,黄金莲.PMBP缩2-氨基苯并噻唑席夫碱/离子液体双水相体系萃取废水中重金属离子的研究[J].冶金分析,2010,30(5): 33-37. http://www.cnki.com.cn/Article/CJFDTOTAL-YJFX201005012.htm
[17] 朱陈银.离子液体双水相中金属离子的萃取分离及测定[D].淮北:淮北师范大学,2010: 22-26.
[18] 沈兴海,徐超,刘新起,褚泰伟.离子液体在金属离子萃取分离中的应用[J].核化学与放射化学,2006,28(3): 129-138. http://cdmd.cnki.com.cn/Article/CDMD-10504-2010010638.htm
[19] 李长平,辛宝平,徐文国.离子液体对Cu(Ⅱ)和Ni(Ⅱ)的萃取性能[J].大连海事大学学报,2008,34(3): 18-20. http://www.cnki.com.cn/Article/CJFDTOTAL-DLHS200803004.htm
[20] 黄治中,罗六宝,张世誉,赵利群.邻二氮菲分光光度法测定铁条件选择实验的改进[J].化工科技,2008,16(6): 50-53. http://www.cnki.com.cn/Article/CJFDTOTAL-JKGH200806020.htm
[21] Visser A E, Swatloski R P, Reichert W M, Griffin S T, Rogers R D. Traditional extractants in nontraditional solvents: Groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids [J].Industrial & Engineering Chemistry Research,2000,39(10): 3596-3604.
-



 下载:
下载: