Numerical experiment on the mechanism of mixing corrosion of carbonate rocks by hydrothermal synergistic effect
-
摘要:
在封闭岩溶水系统中,当水化学组分或温度不同的饱和地下水发生混合时,将增加地下水的碳酸盐矿物溶解度,产生新的溶蚀能力。为了揭示常见地下水混合情况下的饱和溶液混合溶蚀和温度混合溶蚀协同作用机理,文章采用水文地球化学软件PHREEQC模拟了土壤入渗水与浅层地下水、深循环热水与浅层地下水、深部流体与浅层地下水混合条件下的碳酸钙溶蚀反应,讨论了地下水温度、二氧化碳分压(
$ {P}_{{\text{CO}}_{\text{2}}} $ $ {P}_{{\text{CO}}_{\text{2}}} $ $ {P}_{{\text{CO}}_{\text{2}}} $ Abstract:Karst water systems can be divided into open systems and closed systems. In the open system, mixed groundwater dissolves carbonate minerals mainly by the unsaturated corrosion. However, in the closed system, groundwater has been saturated with carbonate minerals after long-term runoff flow. When saturated groundwater with different hydrochemical components or at different temperatures mixed, the solubility of carbonate minerals in groundwater increases, and new corrosion capacity of groundwater generates. The mixing corrosion of saturated solution and temperature mixing corrosion are important dynamics for the chemical dissolution of carbonate aquifer system. The existing research about the interaction mechanism of mixing corrosion of carbonate rocks mainly focuses on the mixing corrosion of single saturated solution or temperature mixing corrosion, and the mechanism of hydrothermal synergistic dissolution in different groundwater mixing situations needs to be further studied. In order to reveal the synergistic mechanism between mixing corrosion of saturated solution and temperature mixing corrosion, this study uses hydro-geochemistry software PHREEQC to simulate the dissolution of calcium carbonate under static mixing conditions of groundwater, including the mixing of infiltrated soil water and shallow groundwater, the mixing of deep-flowing geothermal groundwater and shallow groundwater, and the mixing of deep fluid and shallow groundwater. Moreover, the impact of temperatures,
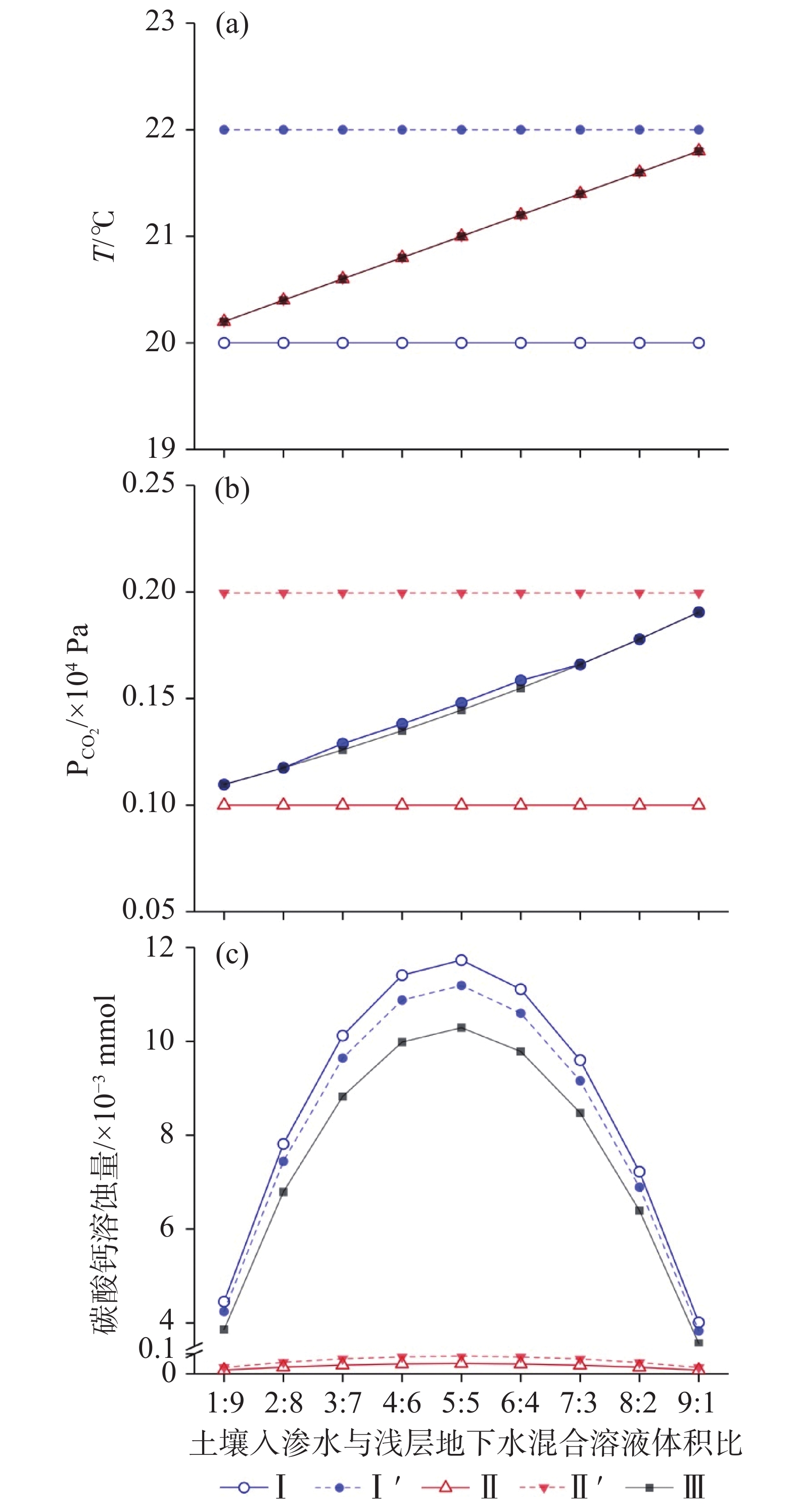
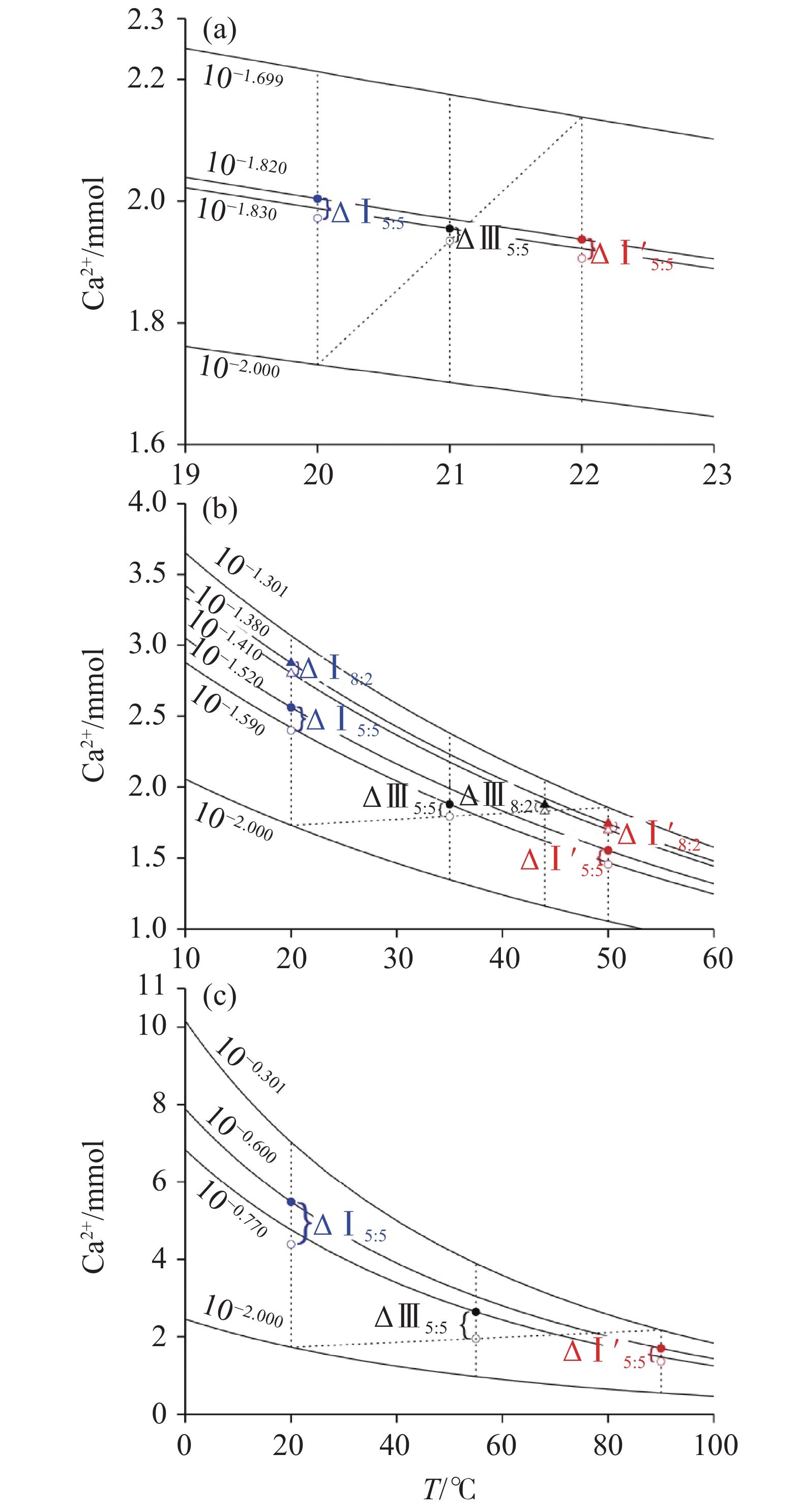
$ {P}_{{\text{CO}}_{\text{2}}} $ The results show, (1) The mixing corrosion by hydrothermal synergistic effect can enhance the supplementary corrosion capacity of saturated groundwater which descends in order as the mixing of deep fluid and shallow groundwater, the mixing of deep-flowing geothermal water and shallow groundwater, and the mixing of infiltrated soil water and shallow groundwater. (2) When the mixing of infiltrated soil water and shallow groundwater occurs, the mixing corrosion capacity by hydrothermal synergistic effect is lower than that of mixing corrosion of single saturated solution, but higher than that of single temperature mixing corrosion. The main reason is that the change of solution temperature weakens the complementary corrosion capacity of mixed solution. (3) Under the condition of mixing deep-flowing geothermal groundwater and shallow groundwater, the mixing corrosion of saturated solution in deep-flowing geothermal groundwater has more effects on the hydrothermal synergistic corrosion than the temperature mixing corrosion. (4) When the deep fluid mixes with the shallow groundwater, the corrosion capacity of mixed solution is weaker than that of the mixing corrosion of saturated solution at single low-temperature, but stronger than that of mixing corrosion at single high-temperature. Although the change of temperature in the mixture causes the calcium carbonate precipitation, the mixing corrosion by hydrothermal synergistic effect still dissolves calcium carbonate. (5) The intensity of mixing corrosion by hydrothermal synergistic effect in the karst water system is controlled synchronously by the change of temperature and
$ {P}_{{\text{CO}}_{\text{2}}} $ $ {P}_{{\text{CO}}_{\text{2}}} $ -
Key words:
- carbonate rock /
- groundwater mixing /
- temperature /
- partial pressure of carbon dioxide /
- mixing ratio
-

-
表 1 碳酸钙混合溶蚀端元溶液热化学参数
Table 1. Thermochemical parameters of mixing corrosion of end-member solution of calcium carbonate
溶液
编号溶液
类型温度/(℃) $ {P}_{{\text{CO}}_{\text{2}}} $ /(×104 Pa)SIc 文献取值 试验值 文献取值 试验值 文献取值 试验值 1 浅层地下水 多年平均气温[26] 20 0.05~0.19[27] 0.1 −0.19~0.15[27] 0 2 土壤入渗水 略高于地下水[28] 22 0.05~0.4[29] 0.2 −1.96~1.21[29] 0 3 深循环热水 32~136[19, 20] 50 0.05~0.6[19] 0.5 0.10~0.18[19] 0 4 深部流体 42~185[30] 90 2.6~7.2[25] 5 −0.2~0.66[25] 0 注:SIc为溶液中CaCO3饱和指数。
Note: SIc is the saturation index of CaCO3 in solution表 2 碳酸钙水热协同混合溶蚀作用数值试验方案
Table 2. Numerical test scheme of mixing corrosion of calcium carbonate by hydrothermal synergistic effect
模式 温度 $ {P}_{{\text{CO}}_{\text{2}}} $ 饱和溶液混合溶蚀作用
(TA=TB,PA≠PB)Ⅰ TA=TB=T1 PA=P1
PB=P2、P3、P4Ⅰ′ TA=TB=T2、T3、T4 温度混合溶蚀作用
(TA≠TB,PA=PB)Ⅱ TA=T1
TB=T2、T3、T4PA=PB=P1 Ⅱ′ PA=PB=P2、P3、P4 饱和溶液混合溶蚀和温度混合溶蚀协同作用
(TA≠TB,PA≠PB)Ⅲ TA=T1
TB=T2、T3、T4PA=P1
PB=P2、P3、P4注:T表示温度,P表示 $ {P}_{{\text{CO}}_{\text{2}}} $ ,下标A、B表示混合条件下的两种端元溶液,下标1、2、3、4分别对应表1中对应编号的溶液。
Note: T represents temperature, P represents$ {P}_{{\text{CO}}_{\text{2}}} $ . Subscripts of A and B stand for two end-member solutions in mixing situations. Subscripts of 1, 2, 3 and 4 correspond to the corresponding numbered solutions in Tab. 1. -
[1] 刘再华. 桂林岩溶水文地质试验场岩溶水文地球化学的研究[J]. 中国岩溶, 1992, 11(3): 33-41.
LIU Zaihua. Study on the karst hydrogeochemistry of the Guilin karst hydrogeological experimental site[J]. Carsologica Sinica, 1992, 11(3): 33-41.
[2] 罗孝芹, 张强, 陈丽影, 孟庆鑫, 赵敏. 基于单因子指数法的贵阳市南明河上游区综合水质评价[J]. 地下水, 2016, 38(1): 80-82.
LUO Xiaoqin, ZHANG Qiang, CHEN Liying, MENG Qingxin, ZHAO Min. Nanming river upstream region's comprehensive quality evaluation in Guiyang based on the single factor index method[J]. Ground Water, 2016, 38(1): 80-82.
[3] Audra Philippe, Palmer Arthur N. Research frontiers in speleogenesis. Dominant processes, hydrogeological conditions and resulting cave patterns[J]. Acta Carsologica, 2015, 44(3): 315-348.
[4] Goldscheider Nico, Mádl-Szőnyi Judit, Erőss Anita, Schill Eva. Review: Thermal water resources in carbonate rock aquifers[J]. Hydrogeology Journal, 2010, 18(6): 1303-1318.
[5] 任美锷, 刘振中. 岩溶学概论[M]. 北京: 商务印书馆, 1983.
[6] Klimchouk A B. Hypogene speleogenesis[J]. Treatise on Geomorphology, 2013: 220-240.
[7] Dreybrodt W, Romanov D, Kaufmann G. Evolution of isolated caves in porous limestone by mixing of phreatic water and surface water at the water table of unconfined aquifers: A model approach[J]. Journal of Hydrology, 2009, 376(1): 200-208.
[8] 闫志为, 刘辉利, 张志卫. 温度及CO2对方解石、白云石溶解度影响特征分析[J]. 中国岩溶, 2009, 28(1): 7-10.
YAN Zhiwei, LIU Huili, ZHANG Zhiwei. Influences of temperature and CO2 on the solubility of calcite and dolomite[J]. Carsologica Sinica, 2009, 28(1): 7-10.
[9] GONG Xing, HOU Wenjuan, FENG Deluan, LUO Qingzi, Yang Xuegiang. Modelling early karstification in future limestone geothermal reservoirs by mixing of meteoric water with cross-formational warm water[J]. Geothermics, 2019, 77: 313-326.
[10] 陈楠, 梁冰. 硫酸盐溶液中温度对方解石和白云石溶解度的影响[J]. 河海大学学报(自然科学版), 2011, 39(6): 661-664.
CHEN Nan, LIANG Bing. Influences of temperature of sulphate solution on solubilities of calcite and dolomite[J]. Journal of Hohai University (Natural Sciences), 2011, 39(6): 661-664.
[11] 贾立龙, 高莎莎. 沁水盆地南部煤层注CO2后矿物溶解作用模拟[J]. 煤炭技术, 2019, 38(3): 89-91.
JIA Lilong, GAO Shasha. Numerical simulation of mineral dissolution in coal bed after injected CO2 in southern Qinshui basin[J]. Coal Technology, 2019, 38(3): 89-91.
[12] 黄思静, 黄可可, 张雪花. 碳酸盐倒退溶解模式的化学热力学基础: 与CO2有关的溶解介质[J]. 成都理工大学学报(自然科学版), 2009, 36(5): 457-464.
HUANG Sijing, HUANG Keke, ZHANG Xuehua. Chemical thermodynamics foundation of retrograde solubility for carbonate: Solution media related to CO2[J]. Journal of Chengdu University of Technology (Science & Technology Edition), 2009, 36(5): 457-464.
[13] 张文博, 操应长, 远光辉. 不同升温速率下方解石与二氧化碳水溶液作用实验[J]. 油气地质与采收率, 2017, 24(3): 57-65.
ZHANG Wenbo, CAO Yingchang, YUAN Guanghui. Experiment of interaction between calcite and fluid saturated with CO2 under different heating rates[J]. Petroleum Geology and Recovery Efficiency, 2017, 24(3): 57-65.
[14] 钱会, 连珺, 窦妍. 地下水混合作用的碳酸钙溶解沉淀效应[J]. 地球科学与环境学报, 2007, 29(1): 55-65.
QIAN Hui, LIAN Jun, DOU Yan. Mixing effects of groundwater on CaCO3 dissolution and precipitation[J]. Journal of Earth Sciences and Environment, 2007, 29(1): 55-65.
[15] 黄奇波, 覃小群, 程瑞瑞, 李腾芳. 左江中游岩溶峰林区河流交互带水化学特征与控制因素[J]. 水文地质工程地质, 2019, 40(5): 1-8.
HUANG Qibo, QIN Xiaoqun, CHENG Ruirui, LI Tengfang. Hydrochenmical characteristics and control factors of karst hyporheic zones in the karst peak forest region of the middle reaches of the Zuo river[J]. Hydrogeology & Engineering Geology, 2019, 40(5): 1-8.
[16] 张绍云, 周忠发, 殷超, 谢雅婷. 喀斯特地区土壤–洞穴CO2时空迁移变化特征[J]. 土壤通报, 2016, 47(5): 1238-1244.
ZHANG Shaoyun, ZHOU Zhongfa, YING Chao, XIE Yating. Spatial and temporal variation characteristics of soil CO2 in karst area[J]. Chinese Journal of Soil Science, 2016, 47(5): 1238-1244.
[17] Gulley Jason, Martin Jonathan, Moore Paul. Vadose CO2 gas drives dissolution at water tables in eogenetic karst aquifers more than mixing dissolution[J]. Earth Surface Processes and Landforms, 2014, 39(13): 1833-1846.
[18] 程文汉. 广东英德市碳酸盐岩地热田地温场特征和成因[J]. 热带地理, 2013, 33(5): 617-620.
CHEN Wenhan. Geotherm characteristics and genesis of a carbonatite geothermal field in Yingde, Guangdong Province[J]. Tropical Geography, 2013, 33(5): 617-620.
[19] 余琴, 杨平恒, 王长江, 李国, 张宇, 张媚, 谢正兰. 重庆市统景温泉水化学特征及混合作用[J]. 中国岩溶, 2017, 36(1): 59-66.
YU Qin, YANG Pingheng, WANG Changjiang, LI Guo, ZHANG Yu, ZHANG Mei, XIE Zhenglan. Hydrochemical characteristics and mixing effect in Tongjing hot springs of Chongqing[J]. Carsologica Sinica, 2017, 36(1): 59-66.
[20] 袁建飞, 邓国仕, 徐芬, 唐业旗, 李鹏岳. 川西南喜德热田地下水水文地球化学特征[J]. 现代地质, 2017, 31(1): 200-208.
YUAN Jianfei, DENG Guoshi, XU Fen, TANG Yeqi, LI Pengyue. Hydrogeochemical characteristics of groundwater in the Xide geothermal field, southwest Sichuan, China[J]. Geoscience, 2017, 31(1): 200-208.
[21] Ta Mingming, Zhou Xun, Guo Juan, Wang Yuan, Wang Xinyun, Xu Yanqiu. Hydrogeochemical characteristics and formation of the hot springs occurring in the plunging ends of an anticline in Chongqing, eastern Sichuan Basin, China[J]. Environmental Earth Sciences, 2019, 78(15): 14.
[22] 汪啸. 广东沿海典型深大断裂带地热水系统形成条件及水文地球化学特征[D]. 武汉: 中国地质大学(武汉), 2018.
WANG Xiao. Formation conditions and hydrogeochemical characteristics of the geothermal water in typical coastal geothermal field with deep faults, Guangdong Province[D]. Wuhan: China University of Geosciences, Wuhan, 2018.
[23] Licour Luciane. The geothermal reservoir of Hainaut: The result of thermal convection in a carbonate and sulfate aquifer[J]. Geologica Belgica, 2014, 17(1): 75-81.
[24] 刘元晴, 周乐, 李伟, 王新峰, 马雪梅, 吕琳, 邓启军, 陈晨. 山东莱芜盆地碳酸盐岩热液溶蚀特征及水文地质意义[J]. 现代地质, 2019, 34(1): 1-8.
LIU Yuanqing, ZHOU Le, LI Wei, Wang Xinfeng, MA Xuemei, LV Lin, DENG Qijun, CHEN Chen. Characteristics and hydrogeological significance of hydrothermal dissolution in carbonate rocks from Laiwu Basin, Shandong Province[J]. Geoscience, 2019, 34(1): 1-8.
[25] 刘再华, 袁道先, 何师意, 张美良, 张加桂. 地热CO2-水-碳酸盐岩系统的地球化学特征及其CO2来源: 以四川黄龙沟、康定和云南中甸下给为例[J]. 中国科学: 地球科学, 2000, 30(2): 209-214.
[26] 黄奇波, 覃小群, 刘朋雨, 张连凯, 苏春田. 非岩溶水和硫酸参与溶蚀对湘南地区地下河流域岩溶碳汇通量的影响[J]. 地球科学进展, 2017, 32(3): 307-318.
HUANG Qibo, QIN Xiaoqun, LIU Pengyu, ZHANG Liankai, SU Chuntian. The influence of allogenic water and sulfuric acid to karst carbon sink in karst subterranean river in southern Hunan[J]. Advances in Earth Science, 2017, 32(3): 307-318.
[27] 刘再华, Chris GROVES, 袁道先, Joe MEIMAN, 姜光辉, 何师意. 水–岩–气相互作用引起的水化学动态变化研究: 以桂林岩溶试验场为例[J]. 水文地质工程地质, 2003(4): 13-18.
LIU Zaihua, Chris GROVES, YUAN Daoxian, Joe MEIMAN, JIANG Guanghui, HE Shiyi. Study on the hydrochemical variations caused by the water-rock-gas interaction: An example from the Guilin karst experimental site[J]. Hydrogeology & Engineering Geology, 2003(4): 13-18.
[28] 汤云涛, 周忠发, 朱粲粲, 汪炎林, 薛冰清, 范宝祥. 贵州省绥阳县麻黄洞土壤渗透水–洞穴水元素变化特征及气候响应[J]. 水土保持通报, 2019, 39(5): 67-76.
TANG Yuntao, ZHOU Zhongfa, ZHU Cancan, WANG Yanlin, XUE Bingqing, FAN Baoxiang. Characteristics of soil infiltration water-cave water elements and climate response analysis in Mahuang cave of Suiyang county, Guizhou Province [J]. Bulletin of Soil and Water Conservation, 2019, 39(5): 67-76.
[29] 张绍云, 周忠发, 张强, 谢雅婷. 贵州织金洞洞穴CO2的来源及其空间分布特征[J]. 中国岩溶, 2016, 35(3): 307-313.
ZHANG Shaoyun, ZHOU Zhongfa, ZHANG Qiang, XIE Yating. Source of cave CO2 and its spatial attributive characteristics of Zhijin cave in Guizhou Province[J]. Carsologica Sinica, 2016, 35(3): 307-313.
[30] 上官志冠. 滇西实验场区主要活动断裂地球化学特征[J]. 地震地质, 1988, 10(4): 136-144.
SHANGGUAN Zhiguan. Geochemical characteristics of the main active faults in western Yunnan earthquake prediction test site[J]. Seismology and Geology, 1988, 10(4): 136-144.
[31] Gong Xing, Yang Xueqiang, Luo Qingzi, Tang Long. Effects of convective heat transport in modelling the early evolution of conduits in limestone aquifers[J]. Geothermics, 2019, 77: 383-394.
-




 下载:
下载: