Assessment of Carbon Dioxide Sequestration Potential of Ultramafic Rocks in Northwest China
-
摘要:
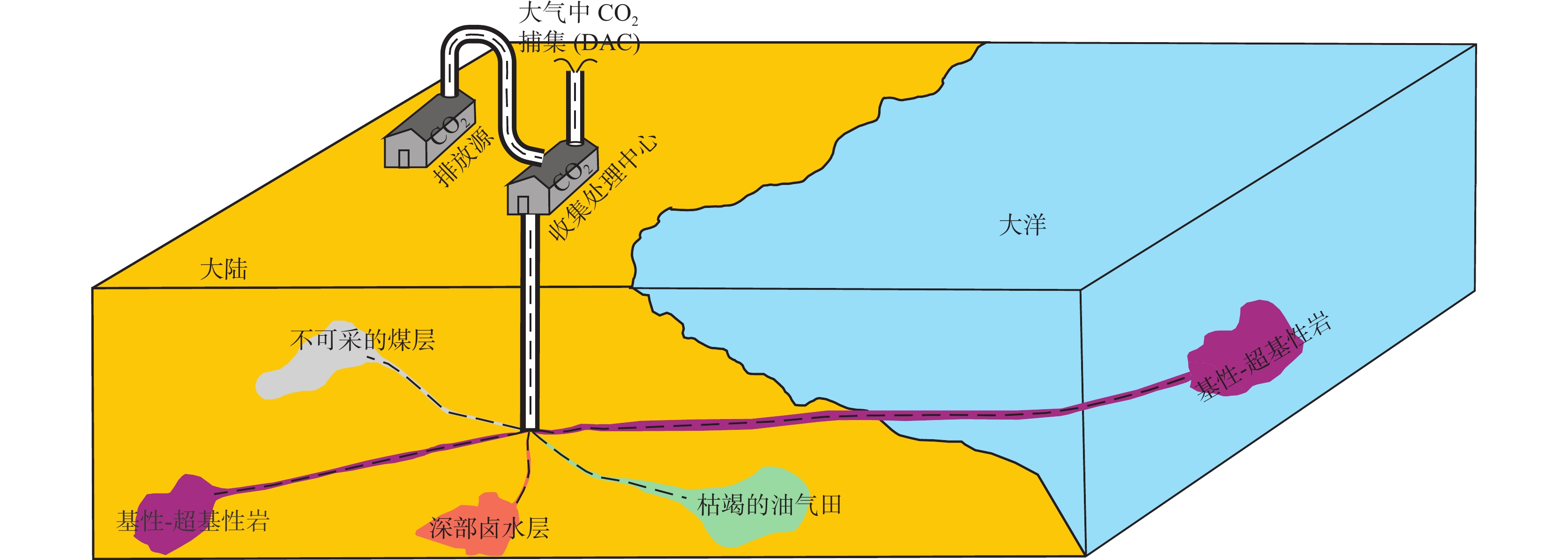
超基性岩可通过碳酸盐化生成稳定的碳酸盐矿物,它是一种以地球化学手段有效且永久封存CO2的矿物。在自然界中矿物封存CO2可通过风化作用自发发生,人工干预能进一步提升碳酸盐化反应效率,促进工业化进程。笔者基于最新1∶100万西北地质图及数据库,试图对西北地区分布的超基性岩的封存潜力进行理论评估。结果表明,西北地区超基性岩封存CO2量可达963.23亿t,其中新疆超基性岩CO2封存量最大,可达613.52亿t,占西北地区总封存量的63.69%。西北地区超基性岩封存CO2量大致相当于全国2021年CO2排放量的10倍,在完全释放其固碳潜力的情况下,初步静态估算可封存全国CO2排放量约10年。因此,西北地区超基性岩封存CO2潜力巨大。未来,应针对单个超基性岩体收集已有大比例尺精细基础地质调查数据,并补充性开展调查及研究工作,进一步圈定CO2地质封存的有利靶区,促进超基性岩封存CO2的地质解决方案成为未来碳中和目标在西北地区落地实现的最优方案之一。
Abstract:Carbonatization of ultramafic rocks is potentially important pathway for carbon mineralization occurred in nature, which is an effective and permanent geochemical trapping system for storing the CO2. The above process can be carried out spontaneously through weathering, and at the same time, manual intervention can further improve the efficiency of carbonatization reaction and promote the process of industrialization. Based on the 1∶1 000 000 geological map and database of northwest China, the storage potential of ultrabasic rocks is evaluated theoretically in this paper. After the statistics and calculations on the data of the ultramafic rocks of the northwest China, the results show that the CO2 sequestration capacity of ultramafic rocks. Totally 96.323 billion tons of carbon sequestration was estimated for northwest China, and the ultramafic rocks in Xinjiang have the greatest amount of CO2 sequestration, which can reach 61.352 billion tons, accounting for 63.69% of the total storage capacity in northwest China. The amount of CO2 sequestered by ultramafic rocks in northwest China is roughly 10 times as against the total CO2 countrywide emissions in 2021. Under the condition that the carbon sequestration potential is fully released, based on the national emissions in 2021, a preliminary static estimate suggest that CO2 can be sequestered for about 10 years. Therefore, ultramafic rocks in northwest China have great potential for CO2 sequestration. In the future, the fine basic geological survey data of the single typical ultramafic rock should be collected, and supplementary survey and research should be carried out to further delineate favorable targets for CO2 sequestration. In this region, the geological solution to promote the sequestration of CO2 in ultramafic rocks has become one of best solutions to achieve the carbon neutrality goals in the future.
-

-
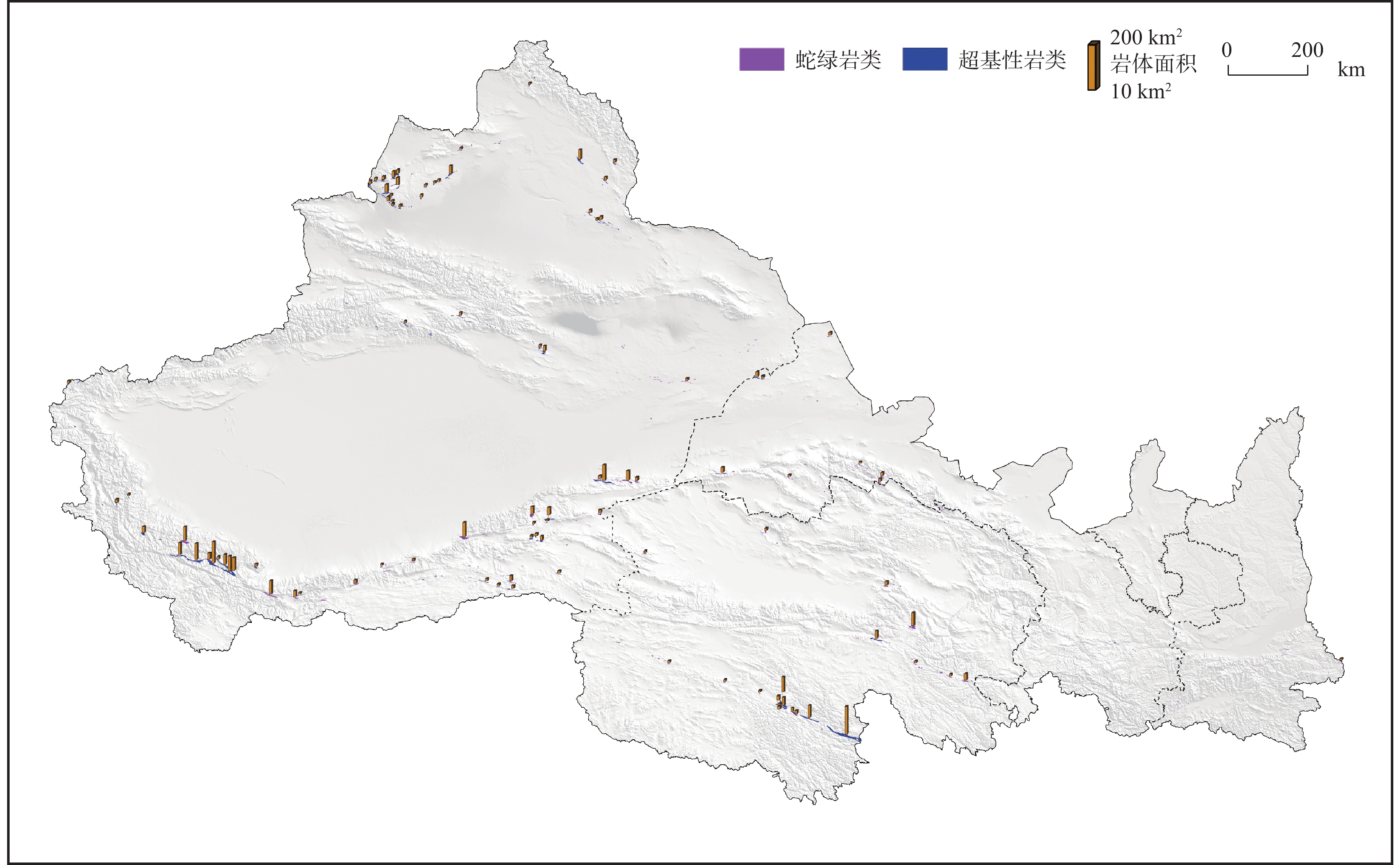
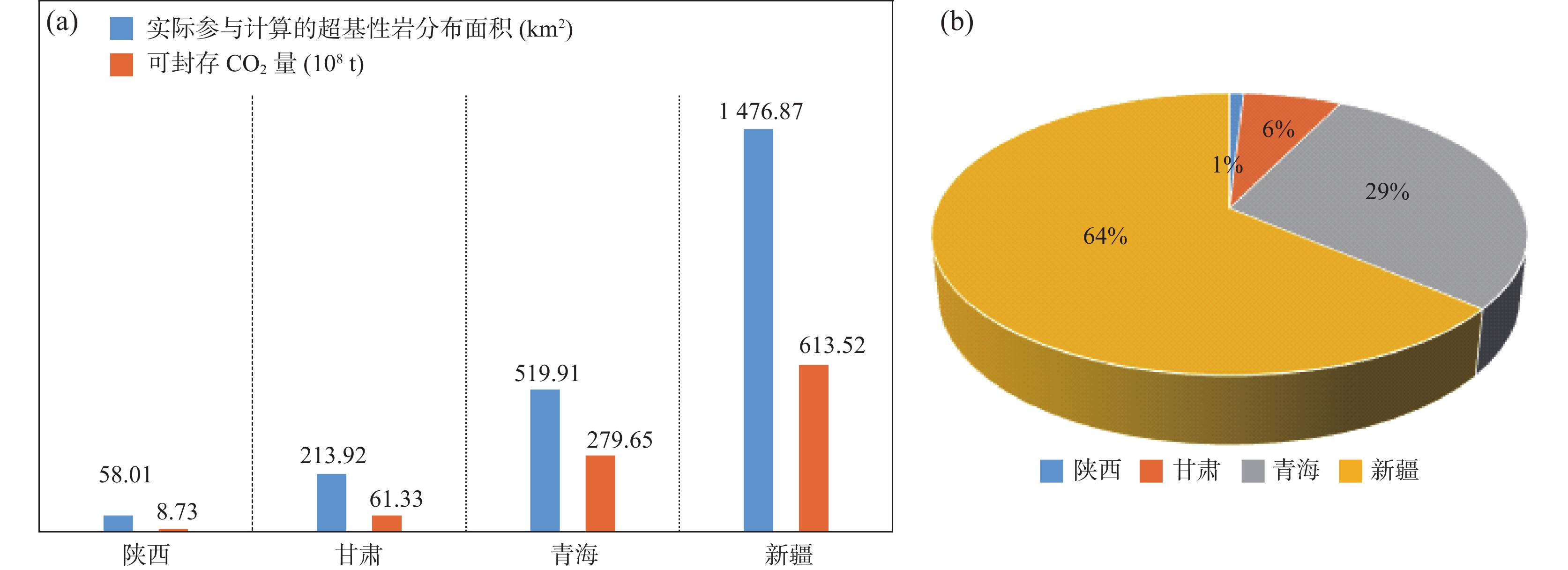
表 1 西北各省(区)超基性岩面积与封存CO2量统计表
Table 1. Distribution area and CO2 storage capacity of ultramafic rocks in the provinces of Northwest China
地区 面积(km2) 各省碳封存量(108t) 西北地区封存总量(108t) 2019年CO2排放量(108t) 可封存时间(年) 百分比(%) 陕西 58.01 8.73 963.23 2.96 2.95 0.91% 甘肃 213.92 61.33 1.64 37.29 6.37% 青海 519.91 279.65 0.52 540.36 29.03% 新疆 1476.87 613.52 4.55 134.76 63.69% -
蔡博峰, 曹丽斌, 雷宇, 等. 中国碳中和目标下的二氧化碳排放路径[J]. 中国人口·资源与环境, 2021, 31(1): 7-14
CAI Bofeng, CAO Libin, LEI Yu, et al. China's Carbon Emission Pathway Under the Carbon Neutrality Target[J]. China Population, Resources and Environment, 2021, 31(1): 7-14.
李智佩, 吴亮, 颜玲丽. 中国西北地区蛇绿岩时空分布与构造演化[J]. 地质通报, 2020, 39(6): 783-817 doi: 10.12097/j.issn.1671-2552.2020.06.002
LI Zhipei, WU Liang, YAN Lingli. Spatial and Temporal Distribution of Ophiolites and Regional Tectonic Evolution in Northwest China[J]. Geological Bulletin of China, 2020, 39(6): 783-817. doi: 10.12097/j.issn.1671-2552.2020.06.002
潘兆橹, 赵爱醒, 潘铁虹. 结晶学及矿物学[M]. 北京: 地质出版社, 1994
PAN Zhaolu, ZHAO Aixing, PAN Tiehong. Crystallography and Mineralogy[M]. Beijing: Geological Publishing House, 1994.
邱添, 曾令森, 申婷婷. 基性-超基性岩碳酸盐化固碳效应研究进展[J]. 中国地质调查, 2021, 8(4): 20-32
QIU Tian, ZENG Lingseng, SHEN Tingting. Progresses on carbon sequestration through carbonation of mafic-ultramafic rocks[J]. Geological Survey of China, 2021, 8(4): 20-32.
盛雪芬, 季峻峰, 陈骏. 中国超基性岩封存CO2的潜力研究[J]. 第四纪研究, 2011, 31(3): 447-454 doi: 10.3969/j.issn.1001-7410.2011.03.07
SHENG Xuefeng, JI Junfeng, CHEN Jun. Assessment of Carbon Dioxides Sequestration Potential of Ultramafic Rocks in China[J]. Quaternary Sciences, 2011, 31(3): 447-454. doi: 10.3969/j.issn.1001-7410.2011.03.07
吴卫华, 郑洪波, 杨杰东, 等. 硅酸盐风化与全球碳循环研究回顾及新进展[J]. 高校地质学报, 2012, 18(2): 215-224 doi: 10.3969/j.issn.1006-7493.2012.02.003
WU Weihua, ZHENG Hongbo, YANG Jiedong, et al. Review and Advancements of Studies on Silicate Weathering and the Global Carbon Cycle[J]. Geological Journal of China Universities, 2012, 18(2): 215-224. doi: 10.3969/j.issn.1006-7493.2012.02.003
张舟, 张宏福. 基性、超基性岩: 二氧化碳地质封存的新途径[J]. 地球科学: 中国地质大学学报, 2012, 37(1): 156-162
ZHANG Zhou, ZHANG Hongfu. Carbonation of Mafic-Ultramafic Rocks: A New Approach to Carbon Dioxide Geological Sequestration[J]. Earth Science-Journal of China University of Geosciences, 2012, 37(1): 156-162.
中华人民共和国生态环境部. 中华人民共和国气候变化第三次国家信息通报[R]. 北京:生态环境部, 2018.
Ministry of Ecology and Environment of the People’s Republic of China. Third National Bulletin of the People’s Republic of China on Climate Change[R]. Beijing: Ministry of Ecology and Environment, 2018
Barnes I, O'Neil J R. The Relationship between Fluids in Some Fresh Alpine-Type Ultramafics and Possible Modern Serpentinization, Western United States[J]. Geological Society of America Bulletin, 1969, 80(10): 1947-1960. doi: 10.1130/0016-7606(1969)80[1947:TRBFIS]2.0.CO;2
Ciotta M, Peyerl D, Zacharias L G L, et al. CO2 storage potential of offshore oil and gas fields in Brazil[J]. International Journal of Greenhouse Gas Control, 2021, 112: 103492. doi: 10.1016/j.ijggc.2021.103492
Gadikota G. Carbon mineralization pathways for carbon capture, storage and utilization[J]. Communications Chemistry, 2021, 4(1): 23. doi: 10.1038/s42004-021-00461-x
Goff F, Lackner K S. Carbon Dioxide Sequestering Using Ultramafic Rocks[J]. Environmental Geosciences, 1998, 5(3): 89-102. doi: 10.1046/j.1526-0984.1998.08014.x
Goldberg D, Slagle A L. A global assessment of deep-sea basalt sites for carbon sequestration[J]. Energy Procedia, 2009, 1(1): 3675-3682. doi: 10.1016/j.egypro.2009.02.165
Gunnarsson I, Aradóttir E, Oelkers EH, et al. The rapid and cost-effective capture and subsurface mineral storage of carbon and sulfur at the CarbFix2 site[J]. International Journal of Greenhouse Gas Control, 2018, 79: 117-126. doi: 10.1016/j.ijggc.2018.08.014
Gutknecht V, Snbjrnsdóttir S S, Sigfússon B, et al. Creating a carbon dioxide removal solution by combining rapid mineralization of CO2 with direct air capture[J]. Energy Procedia, 2018, 146: 129-134. doi: 10.1016/j.egypro.2018.07.017
Harrison A L, Power I M, Dipple G M. Accelerated Carbonation of Brucite in Mine Tailings for Carbon Sequestration[J]. Environmental Science & Technology, 2013, 47(1): 126-134.
Holloway S. An overview of the underground disposal of carbon dioxide[J]. Energy Conversion and Management, 1997, 38(Suppl. ): S193–S198.
IEA. International Energy Agency. World Energy Outlook [R]. 2018.
IEA. The role of CO2 storage [R]. 2019.
Kelemen P B, Matter J. In situ carbonation of peridotite for CO2 storage[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(45): 17295-17300. doi: 10.1073/pnas.0805794105
Mani D, Charan S N, Kumar B. Assessment of carbon dioxide sequestration potential of ultramafic rocks in the greenstone belts of southern India[J]. Current Science, 2008, 94(1): 53-60.
Matter J M, Kelemen P B. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation[J]. Nature Geoscience, 2009, 2(12): 837-841. doi: 10.1038/ngeo683
Matter JM, Stute M, Snæbjörnsdottir SÓ, et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions[J]. Science, 2016, 352(6291): 1312-1314. doi: 10.1126/science.aad8132
McGrail B P, Schaef H T, Ho A M, et al. Potential for carbon dioxide sequestration in flood basalts[J]. Journal of Geophysical Research Solid Earth, 2006, 111: 1-13.
Mervine E M, Wilson S A, Power I M, et al. Potential for offsetting diamond mine carbon emissions through mineral carbonation of processed kimberlite: an assessment of De Beers mine sites in South Africa and Canada[J]. Mineralogy and Petrology, 2018: 1-11.
Oelkers E H, Gisiason S R, Matter J. Mineral Carbonation of CO2[J]. Elements, 2008, 4(5): 333-337. doi: 10.2113/gselements.4.5.333
Peter B, Kelemen, Juerg, et al. Rates and Mechanisms of Mineral Carbonation in Peridotite: Natural Processes and Recipes for Enhanced, in situ CO2 Capture and Storage[J]. Annual Review of Earth and Planetary Sciences, 2011, 39(1): 547-576.
Pokrovsky O S, Schott J. Processes at the magnesium-bearing carbonates/solution interface. II. kinetics and mechanism of magnesite dissolution[J]. Geochimica et Cosmochimica Acta, 1999, 63(6): 881-897. doi: 10.1016/S0016-7037(99)00013-7
Schaef H T, Mcgrail B P. Dissolution of Columbia River Basalt under mildly acidic conditions as a function of temperature: Experimental results relevant to the geological sequestration of carbon dioxide[J]. Applied Geochemistry, 2009, 24(5): 980-987. doi: 10.1016/j.apgeochem.2009.02.025
Seifritz W. CO2 disposal by means of silicates[J]. Nature, 1990, 345(6275): 486-486.
Snbjrnsdóttir S, Sigfússon B, Marieni C, et al. Carbon dioxide storage through mineral carbonation[J]. Nature Reviews Earth & Environment, 2020, 1(2): 90-102.
Suekane T, Nobuso T, Hirai S, et al. Geological storage of carbon dioxide by residual gas and solubility trapping[J]. International Journal of Greenhouse Gas Control, 2008, 2(1): 58-64. doi: 10.1016/S1750-5836(07)00096-5
Vishal V, Verma Y, Chandra D, et al. A systematic capacity assessment and classification of geologic CO2 storage systems in India[J]. International Journal of Greenhouse Gas Control, 2021, 111: 103458. doi: 10.1016/j.ijggc.2021.103458
Wilson S A, Dipple G M, Power I M, et al. Carbon Dioxide Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada[J]. Economic Geology, 2009, 104(1): 95-112. doi: 10.2113/gsecongeo.104.1.95
Zhang S, Depaolo D J. Rates of CO2 Mineralization in Geological Carbon Storage[J]. Accounts of Chemical Research, 2017, 50(9): 2075-2084. doi: 10.1021/acs.accounts.7b00334
-



 下载:
下载: