Hydrochemical Characteristics and Genesis of Groundwater in the Hanzhong Basin
-
摘要:
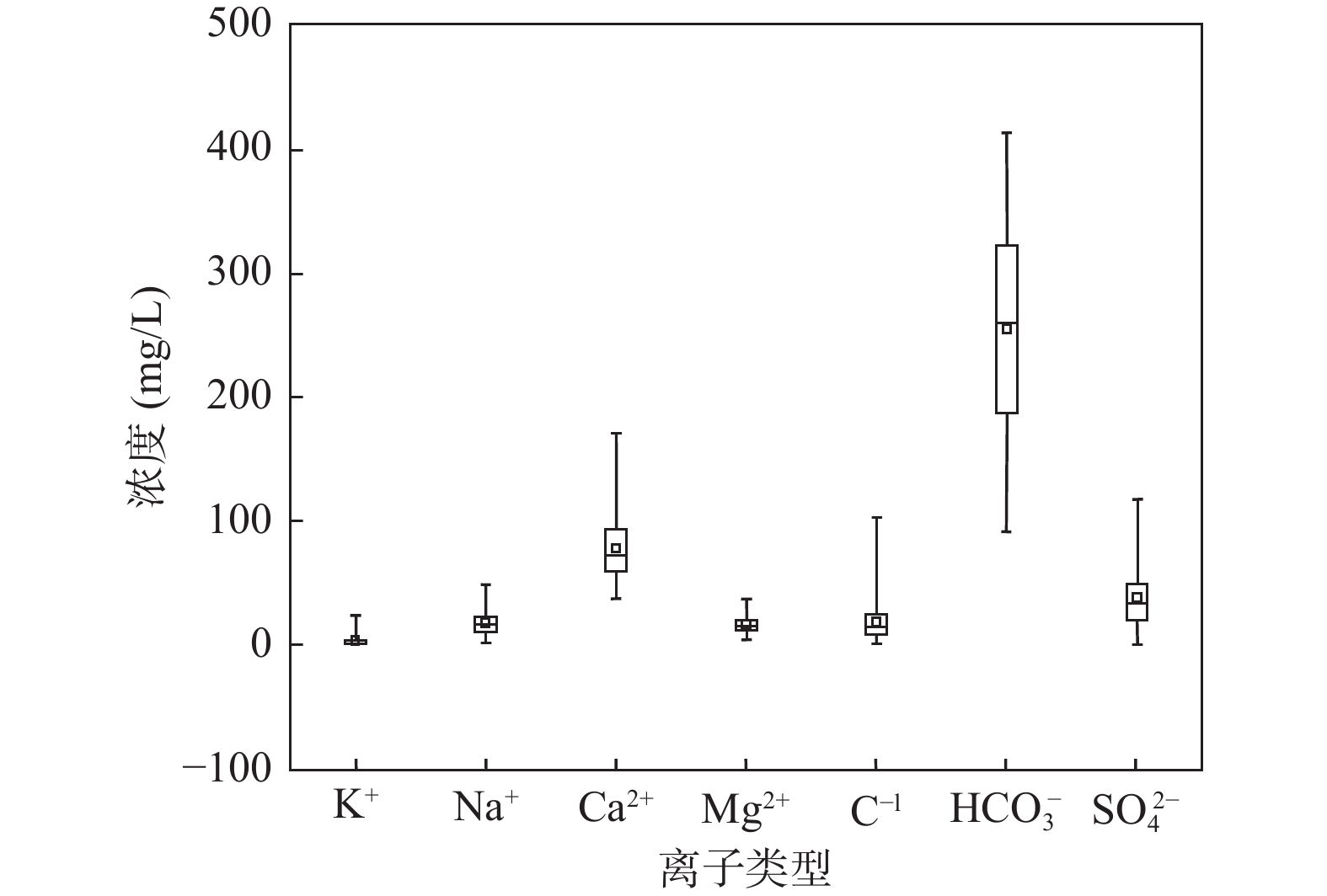
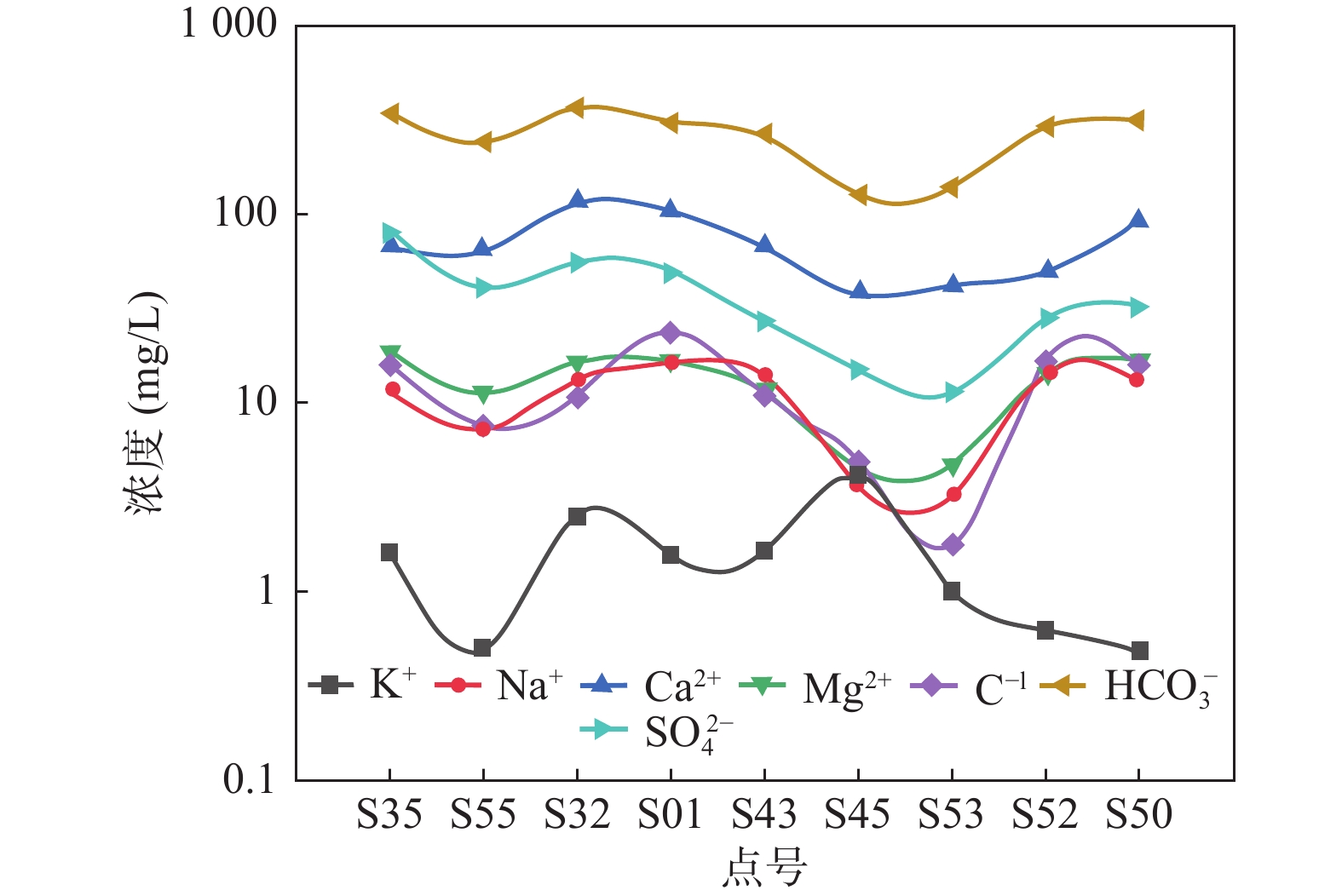
为研究汉中盆地地下水的水化学特征及成因,在丰水期共采集56件潜水样品,综合运用数理统计、相关性分析、Piper三线图、Gibbs模型和离子比等方法,分析了汉中盆地地下水的水文地球化学特征、各化学参数的空间变化规律、主要离子的控制因素及来源。结果表明,汉中盆地地下水阳离子以Ca2+为主,阴离子以HCO3–为主。从离子空间变化规律上来看, K+波动最为剧烈,且从中下游开始逐渐降低,Cl−和Na+变化规律一致,呈波动变化;HCO3−、Ca2+、Mg2+以及SO42−的从中上游至中下游含量逐步降低,至下游含量增加;TDS值为128.5~590 mg/L,平均值为282.67 mg/L,在中下游含量明显增加;pH平均值为7.17,为弱碱性,在中上游波动剧烈,至下游逐渐降低。地下水水化学类型以HCO3–Ca和HCO3–Ca·Mg型为主,受碳酸盐岩和硅酸盐岩的溶解共同控制,阳离子交替吸附作用较弱,Na+和K+主要来源于钾长石、钠长石等硅铝酸盐矿物的溶解和部分盐岩的风化溶解,Ca2+除来自碳酸盐岩的溶解外,还有大量硅酸盐岩的溶解;方解石及少量白云石等碳酸盐岩的风化溶解对Mg2+和HCO3−贡献较大。查明汉中盆地地下水水化学特征及成因机制,可为汉中地区地下水饮水安全提供有力科学依据。
Abstract:In order to study the hydrochemical characteristics and genesis of the groundwater in the Hanzhong Basin, 56 unconfined groundwater samples were collected during the high water period to analyze the hydrogeochemical characteristics of groundwater, the spatial variation of each chemical parameter and control factors and sources of major ions by means of mathematical statistics, correlation analysis, Piper triangular diagrams, Gibbs model and ion ratio and other methods. The results show that Ca2+ is the main cation and the main anion is dominated by HCO3–. From the perspective of spatial variation, K+ fluctuates most violently, and gradually decreases from the middle and downstream. While Cl− and Na+ have the same change law, showing a fluctuation variation. The contents of HCO3−, Ca2+, Mg2+ and SO42− decrease gradually from the middle and upper reaches to the middle and lower reaches, and increase in the lower reaches. TDS ranges from 128.5 mg/L to 590 mg/L, with an average of 282.67 mg/L. The average value of pH is 7.17, showing weakly alkaline, which fluctuates violently in the middle and upper reaches, and gradually decreases in the lower reaches. The hydrochemical types of groundwater are mainly HCO3–Ca and HCO3–Ca·Mg, which are jointly controlled by the dissolution of carbonate rock and silicate rock. The cationic alternate adsorption is weak. Among all the chemical composition of groundwater, Na+ and K+ mainly come from the dissolution of aluminosilicate minerals such as potash feldspar and albite, and the weathering and dissolution of some salt rocks. In addition to the dissolution of carbonate rocks, Ca2+ also comes from the dissolution of a large amount of silicate rocks. Weathering and dissolution of carbonate rocks such as calcite and a small amount of dolomite have a greater contribution to Mg2+ and HCO3−. Identifying the hydrochemical characteristics and genetic mechanism of groundwater in the Hanzhong Basin can provide a strong scientific basis for groundwater drinking water safety in the Hanzhong area.
-
Key words:
- Hanzhong basin /
- groundwater /
- hydrochemistry /
- Piper triangular diagrams /
- chemical weathering
-

-
表 1 主要离子质量浓度统计表(mg/L)
Table 1. Statistics of major irons in groundwater (mg/L)
项目 K+ Na+ Ca2+ Mg2+ Cl− HCO3− SO42− pH TDS 均值 3.53 17.33 77.19 16.23 18.55 253.84 37.75 7.22 292.44 标差 5.48 9.41 25.78 6.70 17.04 86.04 23.89 0.27 152.28 中值 1.40 16.03 72.55 14.85 14.78 259.00 34.10 7.19 262.00 最小值 0.28 2.07 37.50 4.60 0.81 91.50 0.82 6.69 52.00 最大值 23.80 48.50 171.00 36.60 103.00 412.00 117.00 8.19 857.00 注:测试时间为2020年8月。 表 2 各常规指标之间相互关系表
Table 2. Correlation coefficients between major irons in groundwater
K+ Na+ Ca2+ Mg2+ Cl− HCO3− SO42- TDS K+ 1 Na+ 0.282* 1 Ca2+ −0.094 0.427** 1 Mg2+ 0.08 0.312* 0.479** 1 Cl− 0.191 0.789** 0.619** 0.424** 1 HCO3– −0.072 0.003 0.219 0.341* −0.01 1 SO42– 0.217 0.417** 0.499** 0.645** 0.614** 0.12 1 TDS 0.14 0.515** 0.744** 0.410** 0.619** −0.087 0.426** 1 注:*和**分别代表0.05和0.01显著水平。 -
[1] 杜金龙. 潞安矿区中部煤矿补充水源水化学特征及水源识别意义[J]. 西北地质, 2022, 55(1): 208-215
DU Jinlong. Hydrochemical Characteristics of Water Filling Source in Central Lu’an Mining Area and Water Source Identification Significance[J]. Northwestern Geology, 2022, 55(1): 208-215.
[2] 党学亚, 张俊, 常亮, 等. 西北地区水文地质调查与水资源安全[J]. 西北地质, 2022, 55(3): 81−95.
DANG Xueya, ZHANG Jun, CHANG Liang, et al. Hydrogeological Survey and Water Resources Security in Northwest China[J]. Northwestern Geology, 2022, 55(3): 81−95.
[3] 韩朝辉, 朱一龙, 王郅睿, 等. 汉中盆地不同径流条件下地下水水化学特征研究[J]. 地下水, 2022a, 44(1): 26-29 doi: 10.19807/j.cnki.DXS.2022-01-007
HAN Chaohui, ZHU Yilong, WANG Zhirui, et al. Study on Hydrochemical Characteristics of Groundwater under Different Runoff Conditions in Hanzhong Basin[J]. Groung Water, 2022a, 44(1): 26-29. doi: 10.19807/j.cnki.DXS.2022-01-007
[4] 韩朝辉, 朱一龙, 赵超, 等. 汉中盆地西侧土关铺至大安镇一带山区泉水水化学特征及成因机制研究[J]. 吉林大学学报(地球科学版), 2022b, 52(06): 1−11.
HAN Chaohui, ZHU Yilong, ZHAO Chao, et al. Study on the Hydrochemical Characteristics and Genetic Mechanism of Spring Water in the Mountainous Area from Tuguanpu to Da'an Town on the West Side of Hanzhong Basin[J]. Journal of Jilin University (Earth Science Edition), 2022b, 52(06): 1−11.
[5] 王晓曦, 王文科, 王周锋, 等. 滦河下游河水及沿岸地下水水化学特征及其形成作用[J]. 水文地质工程地质, 2014, 41(1): 25-33, 73 doi: 10.16030/j.cnki.issn.1000-3665.2014.01.005
WANG Xiaoxi, WANG Wenke, WANG Zhoufeng, et al. Hydrochemical characteristics and formation mechanism of river water and groundwater along the downstream Luanhe River, northeastern China[J]. Hydrogeology & Engineering Geology, 2014, 41(1): 25-33, 73. doi: 10.16030/j.cnki.issn.1000-3665.2014.01.005
[6] 张帆, 王广才, 张茂省, 等. 产出水识别及受污染地下水水化学和氢氧稳定同位素特征[J]. 西北地质, 2023, 56(3): 98−108.
ZHANG Fan, WANG Guangcai, ZHANG Maosheng, et al. Identification of Produced Water and Characteristics of Hydrochemistry and Stable Hydrogen−Oxygen Isotopes of Contaminated Groundwater[J]. Northwestern Geology, 2023, 56(3): 98−108.
[7] 张俊, 尹立河, 顾小凡, 等. 同位素水化学指示的新疆孔雀河流域地下水与地表水关系[J]. 西北地质, 2021, 54(1): 185−195.
ZHANG Jun, YIN Lihe, GU Xiaofan, et al. Study on the Relationship Between Groundwater and Surface Water in Xinjiang Kongque River Basin Using Isotopes and Hydrochemistry method[J]. Northwestern Geology, 2021, 54(1): 185−195.
[8] 张艳, 吴勇, 杨军, 等. 阆中市思依镇水化学特征及其成因分析[J]. 环境科学, 2015, 36(9): 3230-3237 doi: 10.13227/j.hjkx.2015.09.014
ZHANG Yan, WU Yong, YANG Jun, et al. Hydrochemical characteristic and reasoning analysis in Siyi Town, Langzhong City[J]. Environment Science, 2015, 36(9): 3230-3237. doi: 10.13227/j.hjkx.2015.09.014
[9] 周嘉欣, 丁永建, 曾国雄, 等. 疏勒河上游地表水水化学主离子特征及其控制因素[J]. 环境科学, 2014, 35(9): 3315-3324 doi: 10.13227/j.hjkx.2014.09.011
ZHOU Jiaxin, DING Yongjian, ZENG Guoxiong, et al. Major ion chemistry of surface water in the upper reach of Shule River basin and the possible controls[J]. Environmental Science, 2014, 35(9): 3315-3324. doi: 10.13227/j.hjkx.2014.09.011
[10] 祝虎林. 汉中盆地环境水文地质条件及地下水水质评价[J]. 应用能源技术, 2019, 5: 22-24 doi: 10.3969/j.issn.1009-3230.2019.05.006
ZHU Hulin. Environmental Hydrogeological Conditions and Groundwater Quality Assessment in Hanzhong Basin[J]. Applied Energy Technology, 2019, 5: 22-24. doi: 10.3969/j.issn.1009-3230.2019.05.006
[11] Ayadi Y, Mokadem N, Besser H, et al. Hydrochemistry and stable isotopes (δ18O andδ2H) tools applied to the study of karst aquifers in southern Mediterranean basin (Teboursouk area, NW Tunisia)[J]. Journal of African Earth Sciences, 2018, 137: 208-217. doi: 10.1016/j.jafrearsci.2017.10.018
[12] Bouderbala A, Remini B, Saaed Hamoudi A, et al. Assessment of groundwater vulnerability and quality in coastal aquifers: a case study (Tipaza, North Algeria)[J]. Arabian Journal of Geosciences, 2016, 9(3): 181. doi: 10.1007/s12517-015-2151-6
[13] Chen J S, Wang F Y, Xia X H, et al. Major element chemistry of the Changjiang (Yangtze River)[J]. Chemical Geology, 2002, 187(3-4): 231-255. doi: 10.1016/S0009-2541(02)00032-3
[14] Chetelat B, Liu C Q, Zhao Z Q, et al. Geochemistry of the dissolved load of the Changjiang Basin Rivers: anthropogenic impacts and chemical weathering[J]. Geochimica et Cosmochimica Acta, 2008, 72(17): 4254-4277. doi: 10.1016/j.gca.2008.06.013
[15] Fu C C, Li X Q, Ma J F, et al. A hydrochemistry and multi-isotopic study of groundwater origin and hydrochemical evolution in the middle reaches of the Kuye River basin[J]. Applied Geochemistry, 2018, 98: 82-93. doi: 10.1016/j.apgeochem.2018.08.030
[16] Gibbs R J. Mechanisms controlling world water chemistry[J]. Science, 1970, 170(3962): 1088-1090. doi: 10.1126/science.170.3962.1088
[17] Gibbs R J. Water chemistry of the Amazon River[J]. Geochimica et Cosmochimica Acta, 1972, 36(9): 1061-1066. doi: 10.1016/0016-7037(72)90021-X
[18] Hu M H, Stallard R F, Edmond J M. Major ion chemistry of some large Chinese rivers[J]. Nature, 1982, 298(5): 550-553.
[19] Liu J T, Gao Z J, Wang M, et al. Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China[J]. Environmental Earth Sciences, 2019, 78(3): 1.
[20] Maurya P, Kumari R, Mukherjee S. Hydrochemistry in integration with stable isotopes (δ18O and δD) to assess seawater intrusion in coastal aquifers of Kachchh district, Gujarat, India[J]. Journal of Geochemical Exploration, 2019, 196: 42-56. doi: 10.1016/j.gexplo.2018.09.013
[21] Thomas J, Joseph S, Thrivikramji K. P. Hydrochemical variations of a tropical mountain river system in a rain shadow region of the southern Western Ghats, Kerala, India[J]. Applied Geochemistry, 2015, 63: 456-471. doi: 10.1016/j.apgeochem.2015.03.018
[22] Xiao J, Jin Z D, Wang J, et al. Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Xizang Plateau[J]. Quaternary International, 2015, 380-381: 237-246. doi: 10.1016/j.quaint.2015.01.021
[23] Xiao J, Jin Z D, Zhang F, et al. Major ion geochemistry of shallow groundwater in the Qinghai Lake catchment, NE Qinghai-Xizang Plateau[J]. Environmental Earth Sciences, 2012, 67(5): 1331-1344. doi: 10.1007/s12665-012-1576-4
[24] Yetiş R, Atasoy AD, Demir Yetiş A, et al. Hydrogeochemical characteristics and quality assessment of groundwater in Balikligol Basin, Sanliurfa, Turkey[J]. Environmental Earth Sciences, 2019, 78(11): 331. doi: 10.1007/s12665-019-8330-0
-




 下载:
下载: