Identification of Produced Water and Characteristics of Hydrochemistry and Stable Hydrogen−Oxygen Isotopes of Contaminated Groundwater
-
摘要:
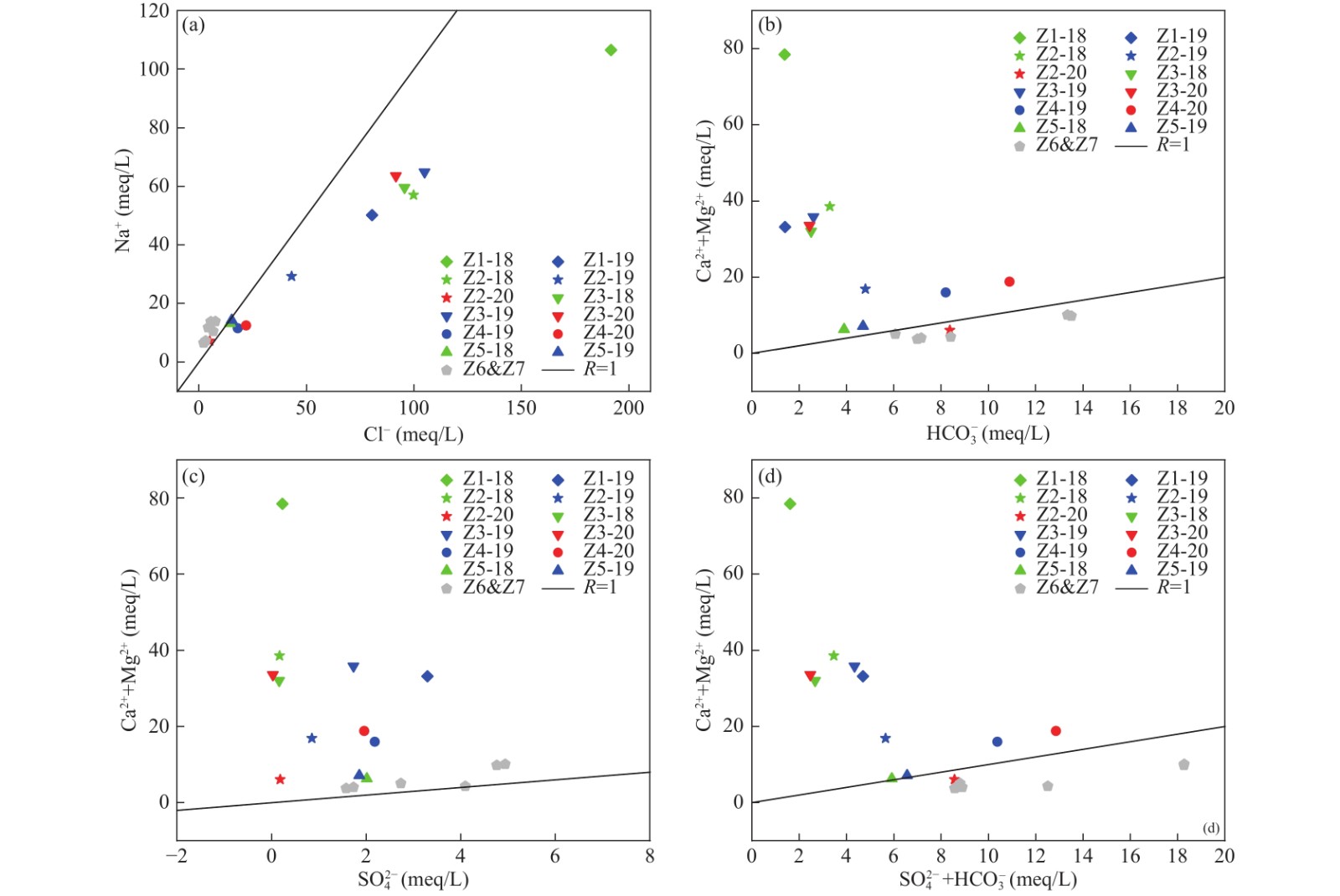
识别地下水污染来源、认识受该类污染源污染的地下水化学特征是地下水污染防治工作的重中之重。产出水作为石油、天然气工业的废水,具有组分复杂、危害性大的特点。针对受产出水污染的地下水研究较少,受污染地下水的特征以及识别该污染源的方法尚不明确的问题,笔者以延安某地下水污染场地为研究区,利用水文地球化学和氢氧稳定同位素的方法探讨受产出水污染的地下水的水化学和同位素特征,并通过对比地下水和油层水的钠氯系数、氯镁系数、脱硫系数和碳酸盐平衡系数对产出水进行识别。研究结果表明,该区域受产出水污染的地下水表现为高TDS和贫化的氢氧稳定同位素特征;其水化学类型以Cl−Na型、Cl−Mg·Ca·Na型为主,且随着受产出水影响程度降低,地下水由Cl−Na型转化为Cl−Mg·Ca·Na型,再到HCO3·SO4−Na·Ca·Mg型;离子比例关系较正常地下水混乱,无线性规律;受产出水污染的地下水的钠氯系数、氯镁系数、脱硫系数和碳酸盐平衡系数大小均在长6油层水的范围内,表明判断油气成藏条件的相关参数可以用来识别产出水污染。该研究探讨了受产出水污染的地下水的水化学特征和氢氧稳定同位素特征,提出对比地下水和油层水的相关参数来识别产出水污染的方法,对产出水污染场地的识别、认识、调查、监测、和修复具有重要意义。
Abstract:Identifying the source of pollution in groundwater and understanding the hydrochemical characteristics of contaminated groundwater by such pollution are very important for pollution prevention of groundwater. As the waste water of petroleum and natural gas industry, the produced water has the characteristics of complex components and great harmfulness. In view of the problems that there was less research on the contaminated groundwater by produced water, and the characteristics of the contaminated groundwater and the method to identify the pollution source were still unclear, the paper toke a polluted groundwater site in Yan’an as the research area, applied the methods of hydrogeochemistry and stable hydrogen−oxygen isotopes to describe the hydrochemical and isotopic characteristics of the groundwater contaminated by produced water, and compared the sodium−chloride coefficients, magnesium−chloride coefficients, desulfurization coefficients and carbonate balance coefficients of groundwater and reservoir water to identify the produced water. The results showed that the contaminated groundwater by produced water in this area was characterized by high TDS and depleted stable hydrogen−oxygen isotope; The chemical types were mainly Cl−Na type and Cl−Mg·Ca·Na type, and with the decrease of the influence of produced water, the groundwater changes from Cl−Na type to Cl−Mg·Ca·Na type and then to HCO3·SO4−Na·Ca·Mg type. The relationship of ion proportions was more chaotic than that of normal groundwater and had no linear law. The sodium−chloride coefficients, magnesium−chloride coefficients, desulfurization coefficients and carbonate balance coefficients of polluted groundwater by produced water were all within the range of Chang 6 reservoir water, indicating that the relevant parameters for judging the conditions of oil and gas accumulation could be used to identify produced water. This study described the hydrochemical and stable hydrogen−oxygen isotopic characteristics of contaminated groundwater by produced water, and proposed a method to identify produced water by comparing the relevant parameters of groundwater and reservoir water, which were of great significance to the identification, recognition, investigation, monitoring and repair of polluted sites by produced water.
-

-
表 1 主要离子和TDS浓度统计表(mg/L)
Table 1. The concentrations of major ions and TDS (mg/L)
样品 时间 Na+ Ca2+ Mg2+ K+ Cl− HCO3− SO42− TDS Z1 2018 2 445 1 467 103 7.78 6 795 84.6 10.9 10 871 2019 1 150 627 37 6.63 2 853 85.4 158.2 4 875 Z2 2018 1 308 702 69 6.11 3 540 201 7.99 5 734 2019 669 312 26 8.04 1 530 293 40.8 2 733 2020 164 106 15.4 3.86 177 511 8.8 731 Z3 2018 1 368 567 73 5.47 3 391 153 7.7 5 488 2019 1 484 668 49 15.91 3 721 159 83.1 6 100 2020 1 460 616 55.8 4.26 3 250 149 1.3 5 462 Z4 2019 262 171 146 5.47 639 500 104.8 1 579 2020 286 207 166 3.31 778 665 94 1 867 Z5 2018 304 99 27 1.53 512 238 96.6 1 180 2019 329 112 30 1.98 542 287 89.1 1 247 Z6 2018 258 18 67 21.48 155 513 196.7 1 003 2019 303 104 90 25.53 195 824 228.6 1 358 2020 307 111 89 21.8 272 814 237 1 445 Z7 2018 241 69 32 2.42 236 370 131.3 912 2019 150 45 29 1.62 78 427 75.6 592 2020 167 51.9 28.4 1.80 110 437 83.2 661 H1 2018 59.5 33.2 18.8 4.66 35 196 59.6 309 2019 135.1 52.4 39.1 10.89 107.2 305.1 133.3 631 2020 144 45.1 34.1 9.99 131 271 138 638 H2 2018 69.3 29.4 18.6 3.54 45.6 196 64.2 329 2019 157.7 41 39.8 10.61 119.2 311.2 145.7 670 2020 149 43.4 33.7 8.63 121 295 143 646 注:Z代表地下水,H代表地表水。 表 2 2018~2020年样品 δD(‰)和 δ18O(‰)值
Table 2. The values of δD (‰) and δ18O (‰) from 2018 to 2020
采样时间 2018年 2019年 2020年 样品 δD(‰) δ18O (‰) δD (‰) δ18O (‰) δD (‰) δ18O (‰) Z1 −84.08 −11.32 −74.78 −9.65 − − Z2 −89.64 −11.74 −80.18 −10.67 −70.65 −9.19 Z3 −84.28 −11.53 −88.33 −12.03 − − Z4 − − −65.86 −8.55 −65.39 −8.60 Z5 −72.78 −9.47 −71.28 −9.84 − − Z6 −64 −8.72 −61.34 −8.43 −63.40 −8.66 Z7 −62.32 −8 −60.81 −8.21 −61.60 −7.69 H1 −53.71 −7.38 −56.08 −7.26 −55.36 −7.30 H2 −51.52 −7.39 −55.99 −6.98 −52.36 −7.13 表 3 理想混合模型计算结果
Table 3. The results of ideal mixing
2018~2019年计算结果(mg/L) 混合分数 Na+ Ca2+ Mg2+ K+ Cl− HCO3− SO42- NO3− 以Na+ 计,x=0.5403 669.01 343.21 48.31 6.91 1 678.80 236.87 67.22 8.77 以Cl− 计,x=0.5835 617.92 314.52 46.66 6.98 1 529.99 239.74 71.96 9.23 实测值 669 312 26 8.04 1 530 293 40.8 1.86 2019~2020年计算结果(mg/L) 混合分数 Na+ Ca2+ Mg2+ K+ Cl− HCO3− SO42- NO3− 以Na+ 计,x=0.9289 164.0 57.43 30.38 7.63 197.26 269.22 112.16 12.77 以Cl− 计,x=0.9430 156.33 53.56 30.44 7.62 177.03 268.86 113.24 12.93 实测值 164 106 15.4 3.86 177 511 8.8 0.14 -
[1] 焦艳军, 王广才, 崔霖峰, 等. 济源盆地地表水和地下水的水化学及氢、氧同位素特征[J]. 环境化学, 2014, 33(06): 962-968 doi: 10.7524/j.issn.0254-6108.2014.06.023
JIAO Yanjun, WANG Guangcai, CUI Linfeng, et al. Characteristics of hydrochemistry and stable hydrogen, oxygen isotopes in surface water and groundwater in Jiyuan Basin[J]. Environmental Chemistry, 2014, 33(06): 962-968. doi: 10.7524/j.issn.0254-6108.2014.06.023
[2] 梁晓伟, 牛小兵, 李卫成, 等. 鄂尔多斯盆地油田水化学特征及地质意义[J]. 成都理工大学学报(自然科学版), 2012, 39(05): 502-508
LIANG Xiaowei, NIU Xiaobing, LI Weicheng, et al. Chemical character of oil-field water in Ordos Basin and geological significance[J]. Journal of Chengdu University of Technology(Science & Technology Edition), 2012, 39(05): 502-508.
[3] 楼章华, 苏一哲, 朱蓉, 等. 四川盆地新场构造带上三叠统须家河组二段地层水化学动态特征及其成因[J]. 石油与天然气地质, 2021, 42(04): 841-851 doi: 10.11743/ogg20210406
LOU Zhanghua, SU Yizhe, ZHU Rong, et al. Dynamic chemical characteristics and origin of formation water in the second member of Xujiahe Formation, Xinchang structural belt, Sichuan Basin[J]. Oil & Gas Geology, 2021, 42(04): 841-851. doi: 10.11743/ogg20210406
[4] 张茂省, 孙传尧, 校培喜, 等. 延安市宝塔区地质灾害详细调查示范[J]. 西北地质, 2007,40(02): 29-55 doi: 10.3969/j.issn.1009-6248.2007.02.002
ZHANG Maosheng, SUN Chuanyao, XIAO Peixi, et al. A Demonstration Project for Detailed Geo-hazard Survey in Baota District, Yan’ an[J]. Northwestern Geology, 2007,40(02): 29-55. doi: 10.3969/j.issn.1009-6248.2007.02.002
[5] 曾溅辉, 吴琼, 杨海军, 等. 塔里木盆地塔中地区地层水化学特征及其石油地质意义[J]. 石油与天然气地质, 2008(02): 223-229 doi: 10.3321/j.issn:0253-9985.2008.02.011
ZENG Jianhui, WU Qiong, YANG Haijun, et al. Chemical characteristics of formation water in Tazhong area of the Tarim Basin and their petroleum geological significance[J]. Oil & Gas Geology, 2008(02): 223-229. doi: 10.3321/j.issn:0253-9985.2008.02.011
[6] 战常武. 曲塘次凹油田地层水特征及其石油地质意义[J]. 承德石油高等专科学校学报, 2020, 22(04): 21-23+34 doi: 10.3969/j.issn.1008-9446.2020.04.006
ZHAN Changwu. Characteristics of Formation Water and Its Petroleum Geological Significance in Qutang Sub Sag[J]. Journal of Chengde Petroleum College, 2020, 22(04): 21-23+34. doi: 10.3969/j.issn.1008-9446.2020.04.006
[7] 张景涛, 史浙明, 王广才, 等. 柴达木盆地大柴旦地区地下水水化学特征及演化规律[J]. 地学前缘, 2021, 28(04): 194-205 doi: 10.13745/j.esf.sf.2020.6.40
ZHANG Jingtao, SHI Zheming, WANG Guangcai, et al. Hydrochemical characteristics and evolution of groundwater in the Dachaidan area, Qaidam Basin[J]. Earth Science Frontiers, 2021, 28(04): 194-205. doi: 10.13745/j.esf.sf.2020.6.40
[8] 张俊, 尹立河, 顾小凡, 等. 同位素水化学指示的新疆孔雀河流域地下水与地表水关系[J]. 西北地质, 2021, 54(01): 185-195 doi: 10.19751/j.cnki.61-1149/p.2021.01.016
ZHANG Jun, YIN Lihe, GU Xiaofan, et al. Study on the Relationship Between Groundwater and Surface Water in Xinjiang Kongque River Basin Using Isotopes and Hydrochemistry method[J]. Northwestern Geology, 2021, 54(01): 185-195. doi: 10.19751/j.cnki.61-1149/p.2021.01.016
[9] 张治波, 刘腾, 李丽荣, 等. 鄂尔多斯盆地CD区块长6地层水化学性质及其地质意义[J]. 矿物岩石, 2017, 37(03): 61-68 doi: 10.19719/j.cnki.1001-6872.2017.03.009
ZHANG Zhibo, LIU Teng, LI Lirong, et al. Chemical Characteristics and Geological Significance of Chang 6 Formation Water in CD Area of Ordos Basin[J]. Journal of Mineralogy and Petrology, 2017, 37(03): 61-68. doi: 10.19719/j.cnki.1001-6872.2017.03.009
[10] 周新平, 邓秀芹, 李士祥, 等. 鄂尔多斯盆地延长组下组合地层水特征及其油气地质意义[J]. 岩性油气藏, 2021, 33(01): 109-120 doi: 10.12108/yxyqc.20210111
ZHOU Xinping, DENG Xiuqin, LI Shixiang, et al. Characteristics of formation water and its geological significance of lower combination of Yanchang Formation in Ordos Basin[J]. Lithologic Reservoirs, 2021, 33(01): 109-120. doi: 10.12108/yxyqc.20210111
[11] 周训, 胡伏生, 何江涛, 等. 地下水科学概论[M]. 北京: 地质出版社, 2014
ZHOU Xun, HU Fusheng, HE Jiangtao, et al. Introduction to Groundwater Science[M]. Beijing: Geological Publishing House, 2014.
[12] Al-Ghouti M A, Al-Kaabi M A, Ashfaq M Y, et al. Produced water characteristics, treatment and reuse: A review[J]. Journal of Water Process Engineering, 2019, 28: 222-239. doi: 10.1016/j.jwpe.2019.02.001
[13] Amakiri K T, Canon A R, Molinari M, et al. Review of oilfield produced water treatment technologies[J]. Chemosphere, 2022, 298: 134064. doi: 10.1016/j.chemosphere.2022.134064
[14] Atekwana E A, Seeger E J. Carbonate and carbon isotopic evolution of groundwater contaminated by produced water brine with hydrocarbons[J]. Applied Geochemistry, 2015, 63: 105-115. doi: 10.1016/j.apgeochem.2015.08.001
[15] Benko K L, Drewes J E. Produced Water in the Western United States: Geographical Distribution, Occurrence, and Composition[J]. Environmental engineering science, 2008, 25(2): 239–246. doi: 10.1089/ees.2007.0026
[16] Chen X L, Sheng Y Z, Wang G C, et al. Microbial compositional and functional traits of BTEX and salinity co-contaminated shallow groundwater by produced water[J]. Water Research, 2022, 215.
[17] Craig H. Isotopic Variations in Meteoric Waters[J]. Science (New York, N. Y. ), 1961, 133(3465): 1702-1703. doi: 10.1126/science.133.3465.1702
[18] Dolan F C, Cath T Y, Hogue T S. Assessing the feasibility of using produced water for irrigation in Colorado[J]. Science of the Total Environment, 2018, 640-641: 619-628. doi: 10.1016/j.scitotenv.2018.05.200
[19] Fakhru’l-Razi A, Pendashteh A, Abdullah L C, et al. Review of technologies for oil and gas produced water treatment[J]. Journal of Hazardous Materials, 2009, 170(2): 530-551.
[20] Huang X J, Wang G C, Liang X Y, et al. Hydrochemical and Stable Isotope (δD and δ18O) Characteristics of Groundwater and Hydrogeochemical Processes in the Ningtiaota Coalfield, Northwest China[J]. Mine Water and the Environment, 2018, 37(1): 119-136. doi: 10.1007/s10230-017-0477-x
[21] Jiang W B, Xu X S, Hall R, et al. Characterization of produced water and surrounding surface water in the Permian Basin, the United States[J]. Journal of Hazardous Materials, 2022, 430.
[22] McDevitt B, McLaughlin M C, Blotevogel J, et al. Oil & gas produced water retention ponds as potential passive treatment for radium removal and beneficial reuse[J]. Environmental science. Processes & impacts, 2021, 23(3): 501-518.
[23] Miller H, Dias K, Hare H, et al. Reusing oil and gas produced water for agricultural irrigation: Effects on soil health and the soil microbiome[J]. Science of the Total Environment, 2020, 722.
[24] Ozgun H, Ersahin M E, Erdem S, et al. Effects of the pre-treatment alternatives on the treatment of oil-gas field produced water by nanofiltration and reverse osmosis membranes[J]. Journal of Chemical Technology & Biotechnology, 2013, 88(8): 1576-1583.
[25] Patterson L A, Konschnik K E, Wiseman H, et al. Unconventional oil and gas spills: risks, mitigation priorities, and state reporting requirements[J]. Environmental science & technology, 2017, 51(5): 2563–2573.
[26] Sanchez-Rosario R, Hildenbrand Z L. Produced Water Treatment and Valorization: A Techno-Economical Review[J]. Energies, 2022, 15(13): 4319.
[27] Shariq L. Health Risks Associated With Arsenic and Cadmium Uptake in Wheat Grain Irrigated With Simulated Hydraulic Fracturing Flowback Water[J]. Journal of Environmental Health, 2019, 81(6): E1-E9.
[28] Shih J S, Saiers J, Anisfeld S, et al. Characterization and analysis of liquid waste from Marcellus Shale gas development[J]. Abstracts of Papers of the Amercian Chemical Society, 2015, 49(16): 9557-9565.
[29] Shores A, Laituri M. The state of produced water generation and risk for groundwater contamination in Weld County, Colorado[J]. Environmental science and pollution research international, 2018, 25(30): 30390-30400. doi: 10.1007/s11356-018-2810-8
[30] Warner N R, Jackson R B, Darrah T H, et al. Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. [J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(30): 11961-11966. doi: 10.1073/pnas.1121181109
[31] Xu B Y, Wang G C. Surface water and groundwater contaminations and the resultant hydrochemical evolution in the Yongxiu area, west of Poyang Lake, China[J]. Environmental Earth Sciences, 2016, 75(3): 1-16.
[32] Zheng Z X, Zhang H D, Chen Z Y, et al. Hydrogeochemical and Isotopic Indicators of Hydraulic Fracturing Flowback Fluids in Shallow Groundwater and Stream Water, derived from Dameigou Shale Gas Extraction in the Northern Qaidam Basin. [J]. Environmental science & technology, 2017, 51(11): 5889-5898.
-




 下载:
下载: