Dispersion Mechanism on Flotation System of Pentlandite and Serpentine in the Presence of Carboxylation Chitosan
-
摘要:
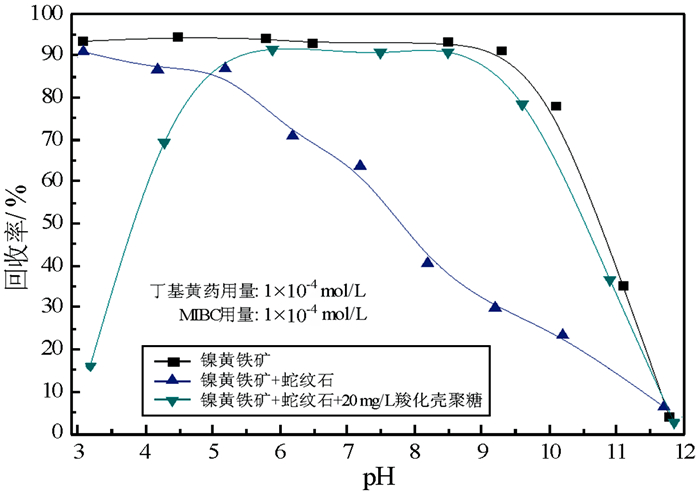
通过矿物浮选试验、沉降试验、动电位和DLVO理论计算,考察了羧化壳聚糖在蛇纹石/镍黄铁矿浮选体系中的聚集/分散作用,研究了羧化壳聚糖对颗粒间的分散作用机理。研究结果表明:蛇纹石颗粒通过异相凝聚罩盖于镍黄铁矿表面上,降低镍黄铁矿表面疏水性能,影响其可浮性;羧化壳聚糖消除了镍黄铁矿与蛇纹石颗粒间异相凝聚,提高镍黄铁矿/蛇纹石体系中镍黄铁矿的浮选回收率。在pH为8.5时,羧化壳聚糖对镍黄铁矿/蛇纹石颗粒间的分散作用显著,并且强于羧甲基纤维素;荷正电的蛇纹石通过静电作用吸附在荷负电镍黄铁矿表面影响其浮选,羧化壳聚糖加入显著改变蛇纹石表面电性,使镍黄铁矿与蛇纹石颗粒间由静电吸引变为静电排斥,表现为异相分散,从而提高镍黄铁矿的浮选回收率。
Abstract:The aggregation/dispersion effect of carboxylation chitosan on flotation system of serpentine and pentlandite and its mechanism were studied by flotation experiments, sedimentation tests, zeta potential, and calculations of DLVO theory. The results show that the serpentine particles coat on the pentlandite surface by hetero-aggregation. It reduces the hydrophobicity of pentlandite surface and decreases the flotation recovery of pentlandite. The hetero-aggregation between pentlandite and the particles of serpentine can be eliminated by adding carboxylation chitosan, which increases the pentlandite recovery in pentlandite/serpentine system. The results show that the effect of carboxylation chitosan on the particles mixture dispersion will be stronger than that of carboxymethyl cellulose at pH 8.5. The positively charged serpentine is absorbed on the negatively charged pentlandite surface by electrostatic interaction, and influences on flotation of pentlandite. The addition of carboxylation chitosan significantly changes the surface electrical properties of serpentine, and makes electrostatic attraction convert into electrostatic repulsion between pentlandite and the particles of serpentine, thereby improving the flotation recovery of pentlandite.
-
Key words:
- serpentine /
- pentlandite /
- carboxylation chitosan /
- dispersion /
- mechanism
-

-
[1] Lu J, Yuan Z, Liu J, et al. Effects of magnetite on magnetic coating behavior in pentlandite and serpentine system[J]. Minerals Engineering, 2015, (72):115-120. http://linkinghub.elsevier.com/retrieve/pii/S0892687514004452
[2] 卢毅屏, 龙涛, 冯其明, 等.微细粒蛇纹石的可浮性及其机理[J].中国有色金属学报, 2009, 19(8):1493-1497. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgysjsxb200908022
[3] Malysiakalysiak V, O'Connor C T, Ralston J, et al. Pentlandite-feldspar interaction and its effect on separation by flotation[J]. International Journal of Mineral Processing, 2002, 66(1-4):89-106. doi: 10.1016/S0301-7516(02)00007-8
[4] Fornasio D, Ralston J. Cu(Ⅱ) and Ni(Ⅱ) activation in the flotation of quartz, serpentine and chlorite[J]. International Journal of Mineral Processing, 2005, 76(1-2):75-81. doi: 10.1016/j.minpro.2004.12.002
[5] Cheng G, Grano S, Sobieraj S, et al. The effect of high intensity conditioning on the flotation of a nickel, part 2: mechanisms[J]. Minerals Engineering, 1999, 12(11):1359-1373. doi: 10.1016/S0892-6875(99)00123-5
[6] 卢毅屏, 张明洋, 冯其明, 等.蛇纹石与滑石的同步抑制原理[J].中国有色金属学报, 2012, 22(2):560-565. https://www.wenkuxiazai.com/doc/1b61a21a6c175f0e7cd137f2.html
[7] 卢毅屏, 丁鹏, 冯其明, 等.不同结构的磷酸盐对蛇纹石的分散作用[J].中南大学学报(自然科学版), 2011, 42(12):3599-3604. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zngydxxb201112001
[8] 冯博, 冯其明, 卢毅屏.羧甲基纤维素在蛇纹石/黄铁矿浮选体系中的分散机理[J].中南大学学报(自然科学版), 2013, 44(7):1933-1939. http://www.cnki.com.cn/Article/CJFDTotal-KCZL201505008.htm
[9] Bremmel L K E, Fornasiero D, Ralston J. Pentlandite-lizardite interactions and implications for their separation by flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 252:207-212. http://cat.inist.fr/?aModele=afficheN&cpsidt=16436554
[10] Wellham E J, Elber L, Yan D S. The role of carboxy methyl cellulose in the flotation of a nickel sulphide transition ore[J]. Minerals Engineering, 1992, 5(3-5):381-395. doi: 10.1016/0892-6875(92)90218-X
[11] Pietrobon M C, Grano S R, Sobieraj S, et al. Recovery mechanisms for pentlandite and MgO-bearing gangue minerals in nickel ores from western Australia[J]. Minerals Engineering, 1997, 10(8):775-786. doi: 10.1016/S0892-6875(97)00056-3
[12] Basile A, Hughes J, McFarlane A J, et al. Development of a model for serpentine quantification in nickel laterite minerals by infrared spectroscopy[J]. Minerals Engineering, 2010, 23(5):407-412. doi: 10.1016/j.mineng.2009.11.018
[13] Kirjavainen V, Heiskanen K. Some factors that affect beneficiation of sulphide nickel-copper ores[J]. Minerals Engineering, 2007, 20 (7):629-633. doi: 10.1016/j.mineng.2007.01.001
[14] Senior G D, Thomas S A. Development and implementation of a new flowsheet for the flotation of a low grade nickel ore[J]. International Journal of Mineral Processing, 2005, 78(1):49-61. doi: 10.1016/j.minpro.2005.08.001
[15] Cao J, Luo Y, Qi L, et al. Utilization of starch graft copolymers as selective depressants for lizardite in the flotation of pentlandite[J]. Applied Surface Science, 2015, 337(8):58-64. http://www.sciencedirect.com/science/article/pii/S016943321500358X
[16] 龙涛, 冯其明, 卢毅屏.六偏磷酸钠在硫化铜镍矿浮选中的分散机理[J].中国有色金属学报, 2012, 22(6):1763-1769. http://www.cnki.com.cn/Article/CJFDTotal-JSKS201508017.htm
[17] Bremmell K E, Fornasiero D, Ralston J. Pentlandite-lizardite interactions and implications for their separation by flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 252:207-212. http://cat.inist.fr/?aModele=afficheN&cpsidt=16436554
[18] Bicak O, Ekmekci Z, Bradshaw D J, et al. Adsorption of guar gum and CMC on pyrite[J]. Minerals Engineering, 2007, 20(10):996-1002. doi: 10.1016/j.mineng.2007.03.002
[19] 丁德润.N-羧甲基壳聚糖对Ca2+, Fe2+的络合(吸附)及光谱分析[J].上海工程技术大学学报, 2004, 18(4):298-301. http://www.whxb.pku.edu.cn/article/2018/1000-6818/WHXB20180207.shtml
[20] Lu Y P, Zhang M Q, Feng Q M, et al. Effect of sodium hexametaphosphate on separation of serpentine from pyrite[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(1):208-213. doi: 10.1016/S1003-6326(11)60701-2
[21] Adamczyk P, Weronski P. Application of the DLVO theory for particle deposition problems[J]. Advances in Colloid and Interface Science, 1999, 83 (1-3):137-226. doi: 10.1016/S0001-8686(99)00009-3
[22] Missnan T, Adell A. On the applicability of DLVO theory to the prediction of clay colloids stability[J]. Journal of Colloid and Interface Science, 2000, 230 (1):150-156. doi: 10.1006/jcis.2000.7003
-



 下载:
下载: