Research on Adsorption Property of Coconut Shell Activated Carbon to Wastewater Containing Sulfur
-
摘要:
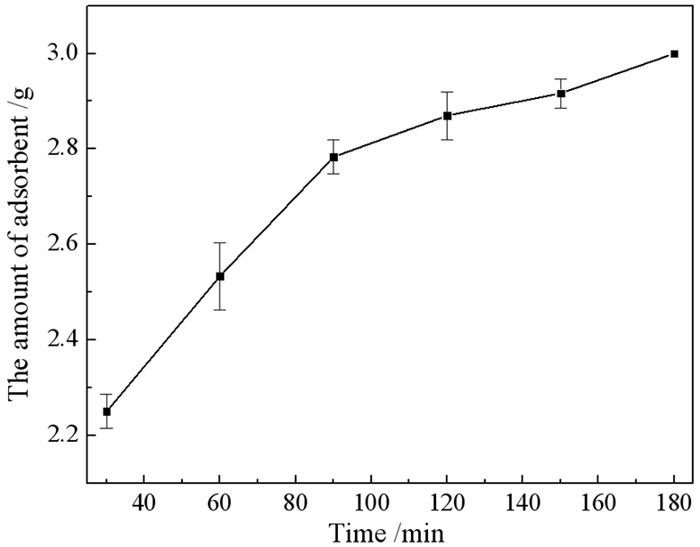
利用椰壳活性炭(CSCC)对铜尾矿氧化焙烧释放的SOx被收集形成的含硫废水进行静态吸附。研究了CSCC对SO42-的吸附处理性能,计算了SO42-的吸附效率,探讨了CSCC的最优使用量、温度、吸附的时间和最优pH值。结果表明:活性炭的最优使用量为2.5 g,最优吸附温度为70 ℃,最优吸附的时间为3 h,最优pH值为7,吸附效率达到96.19%。该研究为CSCC处理含硫废水的实际应用和研究提供了一定的技术参考和依据,对含硫废水的无害化处理和排放有一定的借鉴意义。
Abstract:CSCC is used to statically adsorb the sulfur-containing wastewater formed by the collection of SOx released by the oxidation and roasting of copper tailings. The adsorption treatment performance of CSCC on SO42- was studied, and the adsorption efficiency of SO42- was calculated. The optimal usage, temperature, adsorption time and optimal pH value of CSCC were discussed. Results showed that the adsorption efficiency can reach 96.19% with the activated carbon optimal amount of 2.5 g, the optimal adsorption temperature of 70 ℃, the optimal adsorption time of 3 h, and the optimal pH of 7. This research provides a certain technical reference and basis for the practical application and research of the sulfur-containing wastewater treatment by CSCC. It also has certain reference significance for the harmless treatment and discharge of sulfur-containing wastewater.
-

-
[1] 吴楠, 王三反, 穆永信, 等. 含硫废水处理技术的研究及应用[J]. 广东化工, 2013(8): 100-101. doi: 10.3969/j.issn.1007-1865.2013.08.054
[2] SEKAR M, SAKTHI V, Rengaraj S. Kinetics and equilibrium adsorption study of lead (Ⅱ) onto activated carbon prepared from coconut shell. [J]. Journal of colloid and interface science, 2004, 279(2): 307-313. doi: 10.1016/j.jcis.2004.06.042
[3] 田太福, 关晓彤, 杨旭鹏. 负压抽提法处理含油废水的试验研究[J]. 辽宁化工, 2008, 37(9): 602-604. doi: 10.3969/j.issn.1004-0935.2008.09.008
[4] 郭二亮, 崔雯谣, 吴迪, 等. MnO2/γ-Al2O3的制备及催化空气氧化处理制革含硫废水[J]. 中国皮革, 2019, 48(2): 36-42. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGPG201902008.htm
[5] 董新玉, 郑海华, 万凯. 物化法处理含硫废水的研究进展[J]. 江西化工, 2019(5): 101-103. https://www.cnki.com.cn/Article/CJFDTOTAL-PROV201905035.htm
[6] 孔令瑞. 含硫废水的处理方法研究[J]. 中国食品, 2018(22): 152-153. doi: 10.3969/j.issn.1000-1085.2018.22.068
[7] 李玉红. 含硫污水处理技术研究[J]. 化工管理, 2019(16): 45-45. https://www.cnki.com.cn/Article/CJFDTOTAL-FGGL201916027.htm
[8] GAO J, WANG T, SHU Q, et al. An Adsorption Kinetic Model for Sulfur Dioxide Adsorption by ZL50 Activated Carbon[J]. Chinese journal of chemical engineering, 2010, 18(2): 223-230. doi: 10.1016/S1004-9541(08)60346-8
[9] LIU H, LI W, MA X, et al. Absorbing Low-Concentration Mercaptan with Active Carbon Doped by Copper[J]. Chinese journal of inorganic chemistry, 2016, 32(6): 1026-1032. http://en.cnki.com.cn/Article_en/CJFDTOTAL-WJHX201606013.htm
[10] 吴素强, 刘永, 何鹏. 椰壳活性炭对水溶液中铀(Ⅵ)的吸附研究[J]. 安徽农学通报, 2019, 25(7): 126-129. https://www.cnki.com.cn/Article/CJFDTOTAL-AHNB201907050.htm
[11] 戴一民, 黄璧成. 生物质基多孔炭吸附模拟含硫废水[J]. 明胶科学与技术, 2016, 36(2): 92-99. https://www.cnki.com.cn/Article/CJFDTOTAL-MJKX201602006.htm
[12] AHMAD K S. Adsorption removal of endosulfan through Saccharum officinarum derived activated carbon from selected soils[J]. Journal of central southunivesity, 2019, 26(1): 146-157.
[13] 刘玉德. 椰壳活性炭负载金属氧化物的制备及用于处理染料废水的研究[D]. 南昌: 南昌大学, 2012.
[14] 谭增强, 牛国平, 陈晓文, 等. 椰壳碳基吸附剂的脱汞特性[J]. 环境工程学报, 2015, 9(12): 5992-5996. doi: 10.12030/j.cjee.20151255
[15] 李成龙, 熊泽. 煤质活性炭与椰壳活性炭对漂白废水的吸附性能研究[J]. 化学与生物工程, 2019, 36(2): 51-54. https://www.cnki.com.cn/Article/CJFDTOTAL-HBHG201902012.htm
[16] 邓志华, 刘佩琪, 邓清, 等. 椰壳活性炭对水中重金属离子的吸附研究[J]. 化工新型材料, 2018, 46(3): 273-276. https://www.cnki.com.cn/Article/CJFDTOTAL-HGXC201803068.htm
[17] LUO, B, PENG, T. J, SUN, H. J, et al. Innovative methodology for sulfur release from copper tailings by the oxidation roasting process[J]. J CHEM-NY, 2020, 2020: 1-11. http://www.researchgate.net/publication/342986651_Innovative_Methodology_for_Sulfur_Release_from_Copper_Tailings_by_the_Oxidation_Roasting_Process
[18] 高继贤, 王铁峰, 王金福. SO2体积分数对ZL50活性炭吸附脱硫行为的影响和动力学分析[J]. 环境科学, 2010, 31(5): 1152-1159. https://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201005006.htm
[19] 张建宇, 钟金魁, 赵保卫, 等. 棉花秸秆生物炭对水中硫酸根离子的吸附特性[J]. 环境化学, 2017, 36(11): 2488-2497. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX201711023.htm
[20] WANG Z, SHI M, LI J, et al. Influence of moderate pre-oxidation treatment on the physical, chemical and phosphate adsorption properties of iron-containing activated carbon[J]. Journal of Environmental Sciences, 2014, 26(3): 519-528.
[21] SILVA A M, LIMA R M F, LEAO V A. Mine water treatment with limestone for sulfate removal[J]. Journal of Hazardous Materials, 2012, 221-222: 45-55. http://www.ncbi.nlm.nih.gov/pubmed/22541641
[22] CHANG T, JIE F, Robert K P. Adsorption dynamics of polymeric nanoparticles at an air-water interface with addition of surfactants[J]. Journal of Colloid And Interface Science, 2020, 575: 416-424. http://www.sciencedirect.com/science/article/pii/S0021979720304069
[23] RYU, S. Y, RHIM, J. W, Lee, W. J, . Relationship between Moisture Barrier Properties and Sorption Characteristics of Edible Composite Films[J]. Food Science & Biotechnology, 2005, 14(1): 68-72.
[24] MASZKOWSKA J, WAGIL M, MIODUSZEAWSKA K, et al. Thermodynamic studies for adsorption of ionizable pharmaceuticals onto soil[J]. Chemosphere, 2014, 111: 568-574. http://www.ncbi.nlm.nih.gov/pubmed/24997967?dopt=Abstract
-



 下载:
下载: