The First-principle Study of Silver Activation and Xanthate Adsorption on Sphalerite Surface
-
摘要:
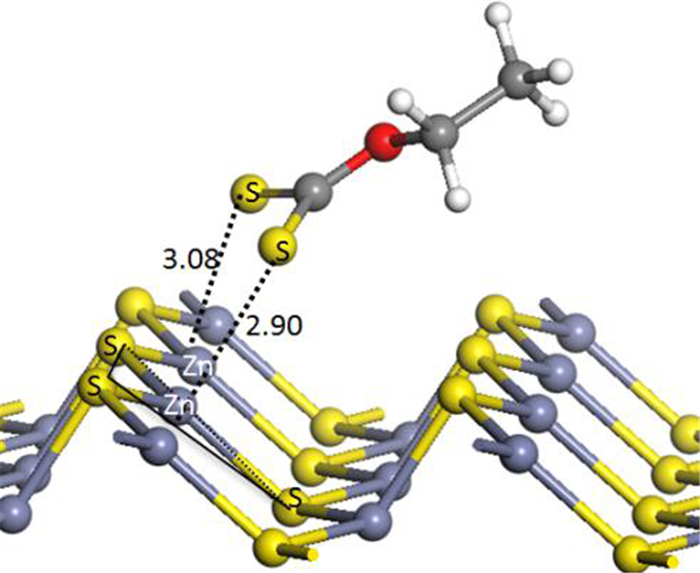
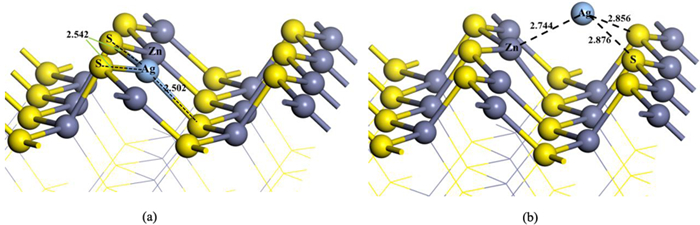
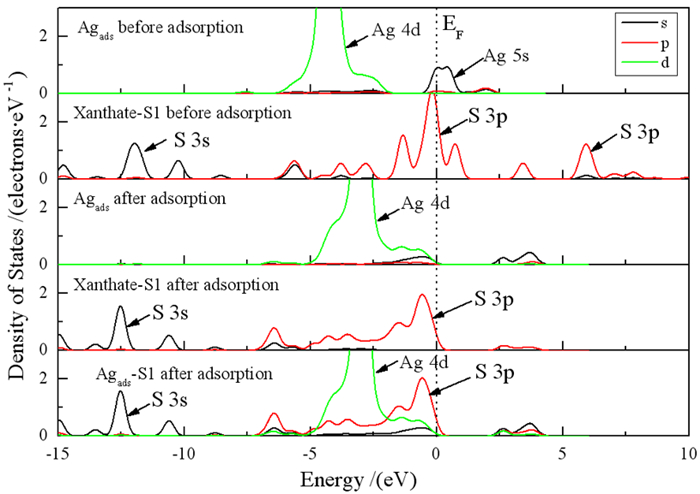
为深入研究银离子对闪锌矿的活化机理,采用第一性原理对闪锌矿表面Ag+替换活化和吸附活化分别开展了模拟研究,同时研究了银活化对闪锌矿表面黄药吸附的影响。计算结果表明,未活化的闪锌矿表面不与乙基黄药发生相互作用,银活化的闪锌矿表面能够与乙基黄药发生相互作用。在两种银活化模型中,Ag+吸附活化比替换活化在能量上更容易发生。Ag+替换活化的闪锌矿表面,黄药的S与表面Ag相互作用较弱;而在Ag+吸附活化的闪锌矿表面,黄药的S与表面Ag相互作用较强。态密度分析表明,在Ag+吸附活化的闪锌矿表面,黄药S原子的3p态和Ag原子的4 d态具有较多成键轨道电子云重叠区域,说明表面吸附的Ag+与黄药的S原子发生了较强的相互作用。Mulliken电荷分析表明,与替换活化相比,黄药和Ag+吸附活化的闪锌矿表面有更多的电荷转移,进一步说明Ag+吸附活化更有可能发生。
Abstract:In order to further study the activation mechanism of silver ions on sphalerite, the Ag+ substitution and adsorption mechanism on sphalerite were studied by density functional theory. Meanwhile, the activation effect of silver to the adsorption of xanthate on sphalerite was studied. The results showed that ethyl xanthate did not interact with the un-activated sphalerite surface but could have strong interaction with the Ag+-activated sphalerite surface. Compare with the two activation models, Ag+ adsorption activation was more likely to occur in terms of energy than Ag+ substitution activation. On the Ag+ substitution-activated sphalerite surface, the S atom of xanthate interacted weakly with surface Ag atom, while on the Ag+ adsorption-activated sphalerite surface, the S atom of xanthate interacted strongly with surface Ag atom. Density of states analysis showed that on Ag+ adsorption-activated sphalerite surface, the 3p state of S atom and the 4d state of Ag atom had many overlapping regions of bonding orbital electron clouds. This indicated that adsorption-activated Ag+ had a strong interaction with S atom of xanthate. Mulliken charge analysis showed that there was more charge transfer between the Ag+ adsorption-activated sphalerite surface and xanthate, which further indicated that Ag+ adsorption-activation was more likely to occur.
-
Key words:
- sphalerite /
- flotation /
- sliver activation /
- the first-principle
-

-
表 1 乙基黄药在银活化闪锌矿表面吸附前后的Mulliken电荷
Table 1. Mulliken charge of ethyl xanthate before and after adsorption on Ag+-activated sphalerite surface
项目 Mulliken电荷/e 吸附前 吸附后 Ag+ (Ag+替换活化模型) 0.37 0.39 乙基黄药S2(Ag+替换活化模型) -0.28 -0.23 Zn(Ag+替换活化模型) 0.47 0.48 S1(Ag+替换活化模型) -0.19 -0.25 Ag+(Ag+吸附活化模型) 0.48 0.33 乙基黄药S1(Ag+吸附活化模型) -0.19 -0.29 -
[1] 曾勇, 刘建, 王瑜, 等. 典型金属离子对闪锌矿浮选行为的影响及作用机制的研究进展[J]. 矿产保护与利用, 2019, 39(2): 109-117. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=1637e6f6-98aa-476f-b4a7-92b9e8d1e394
[2] 胡德勇. 铅锌冶炼科技进步现状与"十五"发展方向[J]. 有色金属工业, 2001(8): 11-13. https://www.cnki.com.cn/Article/CJFDTOTAL-YSGY200108001.htm
[3] 刘建. 闪锌矿表面原子构型及铜吸附活化浮选理论研究[D]. 昆明: 昆明理工大学, 2013.
[4] 黎维中. 难处理铅锌银硫化矿物资源综合回收的研究与实践[D]. 长沙: 中南大学, 2007.
[5] 谢贤. 难选铁闪锌矿多金属矿石的浮选试验与机理探讨[D]. 昆明: 昆明理工大学, 2011.
[6] KARTIO IJ, BASILIO CI, YOONn R-H. An XPS Study of Sphalerite Activation by Copper[J]. American Chemical Society, 1998, 14(18): 0743-7463. http://pubs.acs.org/doi/10.1021/la970440c
[7] CHEN JH, Chen Y, and LI YQ. Quantum-mechanical study of effect of lattice defects on surface properties and copper activation of sphalerite surface[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(6): 1121-1130. doi: 10.1016/S1003-6326(09)60266-1
[8] GERSON AR, LANGE AG, PRINCE KE, SMART RC. The mechanism of copper activation of sphalerite[J]. Applied Surface science, 1999, 137(1): 207-223. http://www.sciencedirect.com/science/article/pii/S0169433298004991
[9] F·拉什齐. 闪锌矿的活化及表面Pb离子浓度[J]. 国外金属矿选矿, 2003, 40(6): 31-36. http://d.wanfangdata.com.cn/Periodical/gwjskxk200306006
[10] FUERSTENAU DW, METZGER PH. Activation of sphalerite with lead ions in the presence of zinc salts[J]. Minerals engineering, 1960, 217: 119-123. http://www.researchgate.net/publication/281198980_Activation_of_sphalerite_with_lead_ions_in_the_presence_of_zinc_salts
[11] POPOV SR, VUINI DR, KAANIK JV. Floatability and adsorption of ethyl xanthate on sphalerite in an alkaline medium in the presence of dissolved lead ions[J]. International Journal of Mineral Processing, 1989, 27(3/4): 205-219. http://www.sciencedirect.com/science/article/pii/0301751689900653
[12] CHEN Y, CHEN JH, and GUO J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite[J]. Minerals engineering, 2010, 23(14): 1120-1130. doi: 10.1016/j.mineng.2010.07.005
[13] REICH M, Becker U. First-principles calculations of the thermodynamic mixing properties of arsenic incorporation into pyrite and marcasite[J]. Chemical Geology, 2006, 225(3/4): 278-290. http://www.sciencedirect.com/science/article/pii/S0009254105003608
[14] CHEN JH, WANG L, CHEN Y, et al. A DFT study of the effect of natural impurities on the electronic structure of galena[J]. International Journal of Mineral Processing, 2011, 98: 132-136. doi: 10.1016/j.minpro.2010.11.001
[15] LONG XH, CHEN JH, and Chen Y. Adsorption of ethyl xanthate on ZnS(110) surface in the presence of water molecules: A DFT study[J]. Applied Surface Science, 2016, 370(5): 11-18.
[16] VANDERBILT, DAVID. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Physical Review B Condensed Matter, 1990, 41(11): 7892. doi: 10.1103/PhysRevB.41.7892
[17] PERDEW JP, Wang Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy[J]. Physical Review B, Condensed matter, 1992, 45(23): 13244-13249. doi: 10.1103/PhysRevB.45.13244
[18] NISHIDATE K, YOSHIZAWA M, HASEGAWA M. Energetics of Mg and B adsorption on polar zinc oxide surfaces from first principles[J]. Physical Review B, Condensed matter, 2008, 77(3): 35330-35336. doi: 10.1103/PhysRevB.77.035330
[19] LEPPINEN JO. FTIR and flotation investigation of the adsorption of ethyl xanthate on activated and non-activated sulfide minerals[J]. International Journal of Mineral Processing, 1990, 30(3): 245-263. http://www.sciencedirect.com/science/article/pii/030175169090018T
-




 下载:
下载: