Influence of Coexisted Ions in Flotation Wastewater on the Ozonation Aniline Aerofloat
-
摘要:
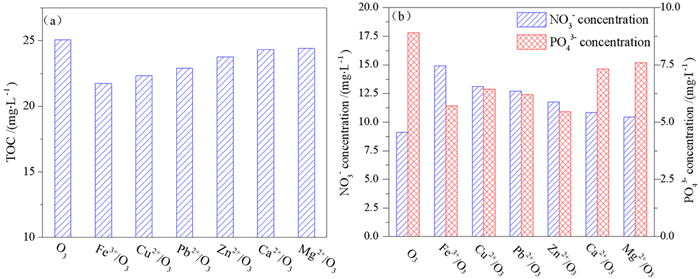
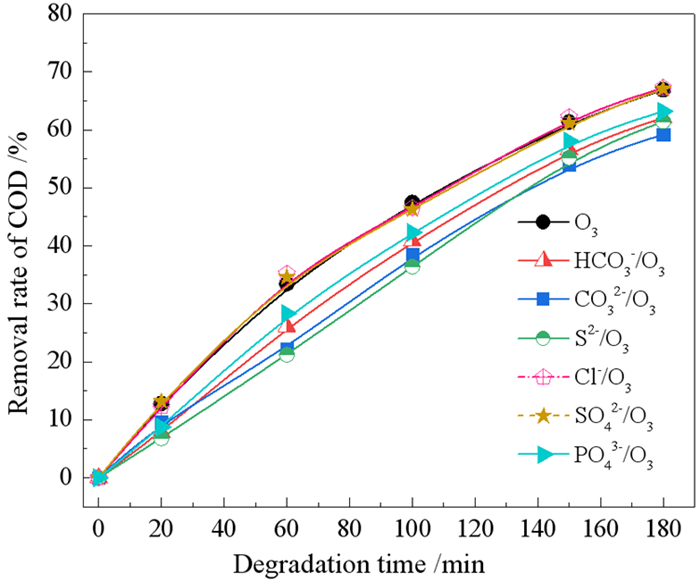
浮选废水中残留有机药剂与各类离子共存,共存离子对臭氧氧化有机药剂有促进或抑制作用,考察了共存离子对臭氧氧化苯胺黑药效率和矿化行为的影响。结果表明,共存阴离子对臭氧氧化苯胺黑药的抑制作用顺序为:CO32- > S2->HCO3- > PO43-,此4种阴离子还可降低苯胺黑药的矿化率,但Cl-和SO42-没有明显的抑制作用;共存金属阳离子对臭氧氧化苯胺黑药的增强作用顺序为:Fe3+ > Cu2+>Pb2+ > Ca2+≈Mg2+ > Zn2+,金属阳离子均能提高苯胺黑药的矿化率,显示出金属离子的催化臭氧氧化性能,而以Fe3+的催化能力最强。Fe3+/O3体系降解苯胺黑药60 min后的有机中间产物种类比单一臭氧氧化少,表明Fe3+的催化作用能强化有机中间产物的再氧化,使苯胺黑药降解过程更为彻底。
Abstract:In flotation wastewater, the residual organic reagents usually coexist with various ions, which can promote or inhibit the ozonation of organic reagents. The effects of coexisting ions on remo FRF-MP-2021val efficiencies of aniline aerofloat (AAF) and its mineralization by the ozonation were investigated. The results indicated that the inhibition order of coexisting anions for the ozonation of AAF was as follows: CO32- > S2- > HCO3- > PO43-. These four anions could also reduce the mineralization rate of AAF, but Cl- and SO42- had no obvious inhibitory effect. The enhancement order of coexisting metallic cations for the ozonation of AAF was as follows: Fe3+>Cu2+ > Pb2+ > Ca2+≈Mg2+ > Zn2+. All of metallic cations could increase the mineralization rate of AAF, which showed the catalytic ozonation capacity of metallic cations. Among them, Fe3+ achieved the highest catalytic capacity. The kinds of organic byproducts in the Fe3+/O3 were much less than that in sole O3 after 60 min degradation of AAF. The results indicated that the catalytic ozonation capacity of Fe3+ could enhance further oxidation of byproducts, leading to more complete degradation of AAF collector.
-
Key words:
- flotation wastewater /
- aniline aerofloat /
- coexisting ions /
- ozonation /
- metallic ions
-

-
表 1 共存阴离子影响臭氧氧化苯胺黑药COD去除的动力学方程
Table 1. Kinetic equations of COD removal in the ozonation of AAF by adding coexisting anions
氧化体系 动力学方程 降解速率常数/min-1 相关性系数 单一O3 ln(Ct/C0)= -0.0061t 0.0061 0.9964 HCO3-/O3 ln(Ct/C0)= -0.0056t 0.0056 0.9981 CO32-/O3 ln(Ct/C0)= -0.0051t 0.0051 0.9947 S2-/O3 ln(Ct/C0)= -0.0055t 0.0055 0.9881 Cl-/O3 ln(Ct/C0)= -0.0061t 0.0061 0.9958 SO42-/O3 ln(Ct/C0)= -0.0060t 0.0060 0.9969 PO43-/O3 ln(Ct/C0)= -0.0058t 0.0058 0.9976 表 2 共存阴离子对臭氧氧化苯胺黑药有机碳、硫、氮和磷的矿化率影响
Table 2. Effect of coexisting anions on the mineralization rates of C, S, N and P in AAF molecule by the ozonation
氧化体系 有机碳的矿化率/% 有机硫的矿化率/% 有机氮的矿化率/% 有机磷的矿化率/% 单一O3 31.14 33.84 18.87 24.36 HCO3-/O3 21.12 19.31 13.45 17.79 CO32-/O3 20.19 23.14 15.44 19.35 S2-/O3 25.79 38.82 16.11 20.34 Cl-/O3 30.05 32.15 17.96 23.13 SO42-/O3 30.46 34.49 18.83 23.95 PO43-/O3 26.5 25.46 17.05 23.46 表 3 金属阳离子影响臭氧氧化苯胺黑药COD去除的动力学方程
Table 3. Kinetic equations of the COD removal in the ozonation of AAF by adding metallic ions
氧化体系 动力学方程 降解速率常数/min-1 相关性系数 单一O3 ln(Ct/C0)=-0.0061t 0.0061 0.994 Fe3+/O3 ln(Ct/C0)=-0.0074t 0.0074 0.987 Cu2+/O3 ln(Ct/C0)=-0.0068t 0.0068 0.991 Pb2+/O3 ln(Ct/C0)=-0.0066t 0.0066 0.986 Zn2+/O3 ln(Ct/C0)=-0.0062t 0.0062 0.988 Ca2+/O3 ln(Ct/C0)=-0.0063t 0.0063 0.993 Mg2+/O3 ln(Ct/C0)=-0.0063t 0.0063 0.996 表 4 金属阳离子对臭氧氧化苯胺黑药有机碳、硫、氮和磷矿化率的影响
Table 4. Effect of metallic ions on the mineralization rates of C, S, N and P in AAF molecule by ozonation
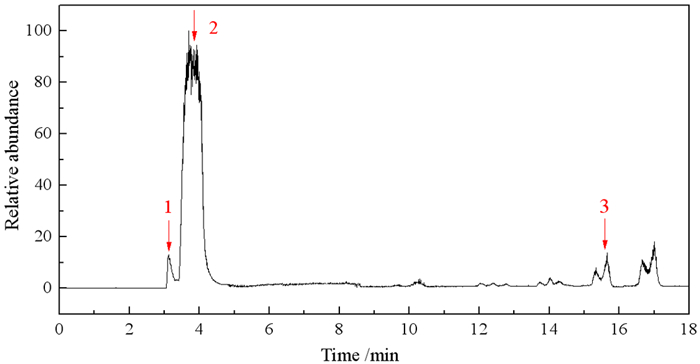
氧化体系 有机碳的矿化率/% 有机硫的矿化率/% 有机氮的矿化率/% 有机磷的矿化率/% 单一O3 31.14 33.84 18.87 24.36 Fe3+/O3 40.29 41.99 31.87 15.65 Cu2+/O3 38.67 41.04 27.82 17.65 Pb2+/O3 37.07 36.54 26.95 17.00 Zn2+/O3 34.68 39.31 24.80 14.94 Ca2+/O3 33.21 35.12 22.86 20.04 Mg2+/O3 32.95 34.68 21.98 20.8 表 5 Fe3+/O3体系降解苯胺黑药60 min时的有机中间产物
Table 5. Organic by products generated from the AAF degraded by the Fe3+/O3 at 60 min
序号 保留时间 分子式 产物 结构式 CAS号 1 3.12 C6H7ON 对氨基苯酚 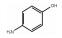
123-30-8 2 3.69 C6H7N 苯胺 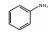
62-53-3 3 15.65 C13H18N6 佐勒汀 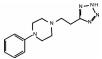
4004-94-8 表 6 单一臭氧氧化苯胺黑药60 min时有机中间产物
Table 6. Organic by products generated from the AAF degraded by sole O3 at 60 min
序号 保留时间 分子式 产物 结构式 CAS号 1 4.68 C11H10 甲基萘 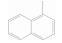
215-329-7 2 6.38 C6H7ON 对氨基苯酚 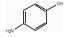
123-30-8 3 7.99 C6H7N 苯胺 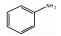
4004-94-8 4 10, 27 C10H14O2 邻二乙氧基苯 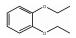
2050-46-6 5 10.99 C15H12 2-甲基蒽 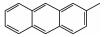
613-12-7 6 11.49 C12H16 环己基苯 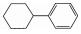
827-52-1 7 12.58 C12H14O 2-苯基环已酮 
1444-65-1 8 14.35 C11H20O4 正丁基丙二酸二乙酯 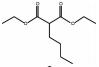
133-08-4 9 15.7 C17H21NO 托莫西汀 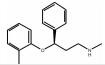
83015-26-3 -
[1] LOTTER N O, BRADSHAW D J. The formulation and use of mixed collectors in sulphide flotation[J]. Minerals Engineering, 2010, 23(11): 945-951. http://www.sciencedirect.com/science/article/pii/S0892687510000671
[2] 董栋. 铅锌选矿废水处理与回用试验研究[D]. 长沙: 中南大学, 2012.
[3] 赵玉娥. 黄药、黑药、二号油在水体中的降解试验研究[J]. 黄金, 1995(7): 47-51. https://www.cnki.com.cn/Article/CJFDTOTAL-HJZZ507.010.htm
[4] 程亚杰, 宋卫锋, 林丽婷, 等. 苯胺黑药的厌氧生物降解与机理[J]. 中国环境科学, 2016, 36(4): 1033-1038. doi: 10.3969/j.issn.1000-6923.2016.04.011
[5] 揭轩敏, 王晖, 陈勇, 等. 螯合混凝沉淀法净化含苯胺黑药废水[J]. 应用化工, 2015, 44(6): 1076-1079. https://www.cnki.com.cn/Article/CJFDTOTAL-SXHG201506025.htm
[6] 祝思频. TiO2基光催化剂降解苯胺黑药的研究[D]. 赣州: 江西理工大学, 2018.
[7] 李洪枚, 王鼎九, 马福洪, 等. 高铁酸钾氧化处理含苯胺黑药废水试验研究[J]. 湿法冶金, 2020(1): 74-79. https://www.cnki.com.cn/Article/CJFDTOTAL-SFYJ202001019.htm
[8] 李韵捷, 梁嘉林, 孙水裕. 臭氧氧化降解苯胺黑药模拟废水[J]. 环境工程学报, 2015, 9(3): 1161-1165. https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201503028.htm
[9] 席彩文, 刘彬彬. 臭氧氧化法处理难降解有机废水[J]. 工业安全与环保, 2005(11): 18-20. https://www.cnki.com.cn/Article/CJFDTOTAL-GYAF200511006.htm
[10] 刘晶冰, 燕磊, 白文荣, 等. 高级氧化技术在水处理的研究进展[J]. 水处理技术, 2011, 37(3): 11-17. https://www.cnki.com.cn/Article/CJFDTOTAL-SCLJ201103004.htm
[11] KUNZ A, REGINATTO V, Durán N. Combined treatment of textile effluent using the sequence phanerochaete chrysosporium-zone[J]. Chemosphere, 2001, 44(2): 281-287. doi: 10.1016/S0045-6535(00)00165-X
[12] NAWROCKI J, HORDERN B K. The efficiency and mechanisms of catalytic ozonation[J]. Applied Catalysis B, Environmental, 2010, 99(1): 27-42. http://www.sciencedirect.com/science/article/pii/S0926337310002833
[13] 林小凤, 傅平丰, 马艳红, 等. 矿物催化臭氧氧化乙硫氨酯的效率和矿化行为研究[J]. 矿产保护与利用, 2020, 40(1): 1-7. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=ebc2ca5e-13f2-44e7-901a-f424bef80c1f
[14] TOMIYASU H, FUKUTMI H, GORDON G. Kinetics and mechanisms of ozone decomposition in basic aqueous solutions[J]. Inorganic Chemistry, 1985, 24(19): 2962-2966. doi: 10.1021/ic00213a018
[15] HOIGNÉ J, BADER H. Rate constants of reactions of ozone with organic and inorganic and inorganic compounds in water: ⅲ. inorganic compounds and radicals[J]. Water Research, 1985, 19(8): 993-1004. doi: 10.1016/0043-1354(85)90368-9
[16] GUNTEN U V. Ozonation of drinking water: part ⅰ. oxidation kinetics and product formation[J]. Water Research, 2003, 37(7): 1443-1467. doi: 10.1016/S0043-1354(02)00457-8
[17] HORDERN B K, Zióek M, Nawrocki J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment[J]. Applied Catalysis B, Environmental, 2003, 46(4): 639-669. doi: 10.1016/S0926-3373(03)00326-6
[18] PIERA E, CALPE J C, BRILLAS E, et al. 2, 4-dichlorophenoxyacetic acid degradation by catalyzed ozonation: TiO2/UVA/O3 and Fe(Ⅱ)/UVA/O3 systems[J]. Applied Catalysis B, Environmental, 2000, 27(3): 169-177. doi: 10.1016/S0926-3373(00)00149-1
[19] WU C H, KUO C Y, CHANG C L. Homogeneous catalytic ozonation of C.I. Reactive Red 2 by metallic ions in a bubble column reactor[J]. Journal of Hazardous Materials, 2008, 154(1-3): 748-755. doi: 10.1016/j.jhazmat.2007.10.087
[20] PINES D S, RECKHOW D A. Effect of dissolved cobalt(Ⅱ) on the ozonation of oxalic acid[J]. Environmental Science & Technology, 2002, 36(19): 4046-4051. http://europepmc.org/abstract/MED/12380073
-



 下载:
下载: