Effect of Size Regulation of Natural Molybdenite on Electrochemical Performance for Lithium-ion Batteries
-
摘要:
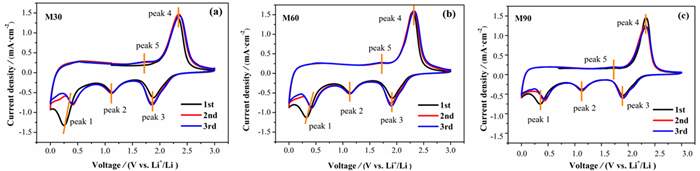
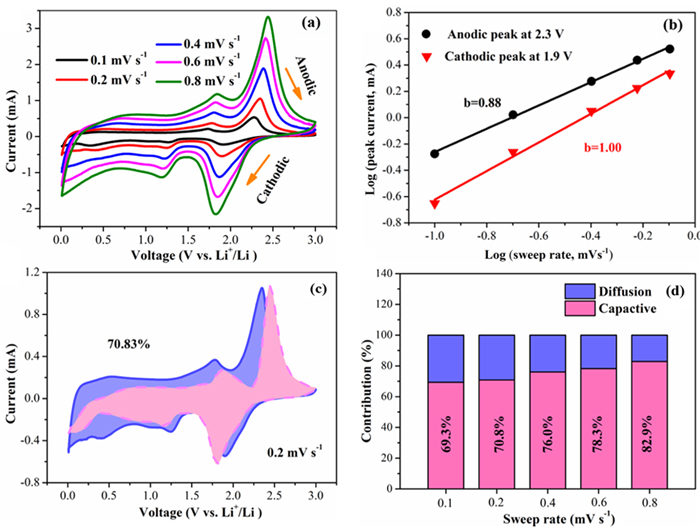
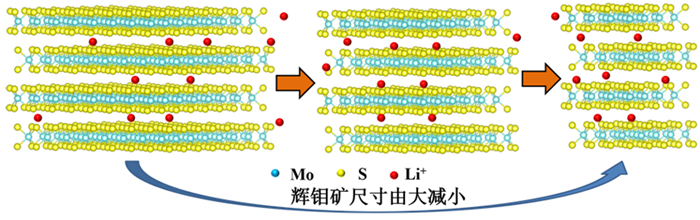
负极材料的尺寸对锂离子电池性能有着重要影响,设计研磨时间制备不同尺寸的天然辉钼矿,探究尺寸与锂离子电池的电化学性能之间的联系。粒度分布仪得出分别研磨30(M30)、60(M60)和90 min(M90)的样品其平均尺寸分别为19.45、13.14和11.23 μm;XRD和SEM表明尺寸越小,晶粒尺寸越小,边缘破碎越严重;电化学性能测试表明,三者首圈容量分别为851、797和649 mAh·g-1,100圈后容量保持率分别为30%、38%和85%。M90具有最大的锂离子扩散系数为3.29×10-10,以0.1~0.8 mV·s-1的不同CV扫描速率计算得出赝电容为主要的容量贡献,可实现电子和离子的快速穿梭。天然辉钼矿的尺寸越小,首圈容量越小,但循环和倍率性能更好和反应动力学更快,并提出类似天然辉钼矿层状储锂模型来阐明天然辉钼矿尺寸与锂离子电池性能关系。
Abstract:The size of anode materials has an essential effect on the performance of lithium-ion batteries. The grinding time was designed to prepare different sizes of natural molybdenite, and the relationship between the size and the electrochemical performance of lithium-ion batteries was explored. The average sizes of the samples abraded for 30 (M30), 60 (M60), and 90 min (M90) were 19.45, 13.14, and 11.23 μm, respectively. XRD and SEM show that the smaller the particle size, the smaller the grain size, and the more serious the edge crushing is. Electrochemical performance tests showed that the first cycle capacities of the three groups were 851, 797, and 649 mAh·g-1, respectively, and the capacity retention rates after 100 cycles were 30%, 38%, and 85%, respectively. M90 had the maximum lithium-ion diffusion coefficient of 3.29×10-10, and the pseudocapacitance was the main contribution to the capacity calculated at different CV scanning rates of 0.1~0.8 mV·s-1, which could realize rapid electron and ion shuttle. The smaller the size of natural molybdenite, the smaller the first cycle capacity, but better cycle and rate performance and faster reaction kinetics, and similar to the natural molybdenite layered lithium storage model were proposed to clarify the relationship between the size of natural molybdenite and the performance of the lithium-ion battery.
-

-
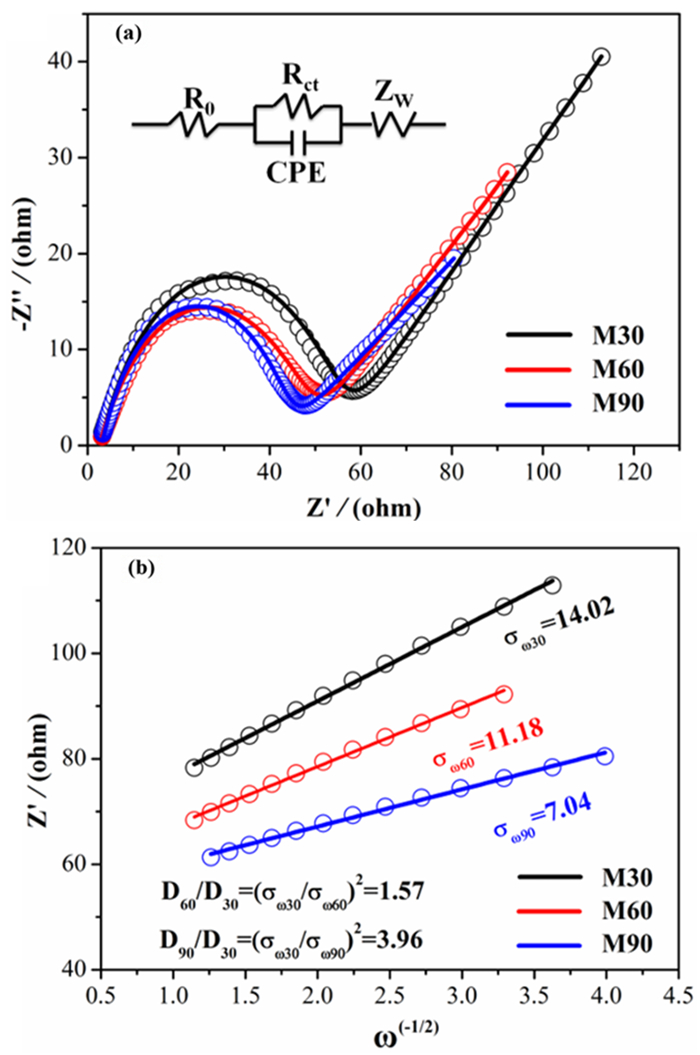
表 1 不同尺寸天然辉钼矿的交流阻抗谱的拟合值
Table 1. Fitting values of alternating current impedance spectra of natural molybdenite with different particle sizes
Electrode M30 M60 M90 Re 2.79 2.59 2.51 Rct 51.44 44.25 38.77 表 2 不同尺寸天然辉钼矿的因子和DLi+值
Table 2. Different granularity of natural molybdenite factor and DLi+ values
Electrode M30 M60 M90 σ 14.02 11.18 7.04 DLi+ 8.31×10-11 1.30×10-10 3.29×10-10 -
[1] BAI J, ZHAO BC, LIN S, et al. Construction of hierarchical V4C3-MXene/MoS2/C nanohybrids for high rate lithium-ion batteries[J]. Nanoscale, 2020, 12(7): 1144-1154. http://pubs.rsc.org/en/content/articlelanding/2020/nr/c9nr07646h
[2] GOODENOUGH, J B. Evolution of strategies for modern rechargeable batteries[J]. Accounts of Chemical Research, 2013, 46(5): 1053-1061. doi: 10.1021/ar2002705
[3] LU XH, YU MH, WANG GM, et al. Flexible solid-state supercapacitors: design, fabrication and applications[J]. Energy Environmental Science, 2014, 7(7): 2160-2181. doi: 10.1039/c4ee00960f
[4] LIANG P, XING S, SHU HB, et al. Three-dimensional MoS2/graphene composites for reversible Li storage[J]. Journal of Inorganic Materials, 2016, 31(6): 575-580. doi: 10.15541/jim20150409
[5] ZHOU Y, LIU Y, ZHAO WX, et al. Growth of vertically aligned MoS2 nanosheets on Ti substrate through self-supported bonding interface for high-performance lithium-ion batteries: a general approach[J]. Journal of Materials Chemistry A, 2016, 4(16): 5932-5941. doi: 10.1039/C6TA01116K
[6] WANG DG, FAN LR, WANG SP, et al. Electrochemical properties of pyrite as lithium battery cathode materials[J]. Materials Review, 2012, 26(18): 93-96. http://search.cnki.net/down/default.aspx?filename=CLDB201218026&dbcode=CJFD&year=2012&dflag=pdfdown
[7] RONG H, WANG CG, ZHOU M. Synthesis and electrochemical performance of FeS2 microspheres as an anode for Li-ion batteries[J]. Chemical Journal of Chinese Universities, 2020, 41(3): 447-455.
[8] SUN D, YE DL, LIU P, et al. MoS2/graphene nanosheets from commercial bulky MoS2 and graphite as anode materials for high rate sodium-ion batteries[J]. Advanced Energy Materials, 2018, 8(10): 1702383. doi: 10.1002/aenm.201702383
[9] LI SJ, TANG HH, GE P, et al. Electrochemical investigation of natural ore molybdentie (MoS2) as a first-hand anode for lithium storages[J]. ACS Applied Materials & Interfaces, 2018, 10(7): 6378-6389. https://pubmed.ncbi.nlm.nih.gov/29376632/
[10] WANG XF, LI YJ, Guan ZRX, et al. Micro-MoS2 with excellent reversible sodium-ion storage[J]. Chemistry, 2015, 21(17): 6465-6468. doi: 10.1002/chem.201406635
[11] VU A, QIAN Y, STEIN A. Porous electrode materials for lithium-ion batteries how to prepare them and what makes them special[J]. Advanced Energy Materials, 2012, 2(9): 1056-1085. doi: 10.1002/aenm.201200320
[12] LUO H, ZHANG LZ, LU Y. Synthesis of MoS2/C submicrosphere by PVP-assisted hydrothermal method for lithium lon battery[J]. Advanced Materials Research, 2012, 531: 471-477. doi: 10.4028/www.scientific.net/AMR.531.471
[13] LIANG SQ, ZHOU J, LIU J, et al. PVP-assisted synthesis of MoS2 nanosheets with improved lithium storage properties[J]. CrystEngComm, 2013, 15(25): 4998-5002. doi: 10.1039/c3ce40392k
[14] CHANG K, CHEN WX. Single-layer MoS2/graphene dispersed in amorphous carbon: towards high electrochemical performances in rechargeable lithium ion batteries[J]. Journal of Materials Chemistry, 2011, 21(43): 17175. doi: 10.1039/c1jm12942b
[15] UTTAM KUMAR SEN, SAGAR MITRA. High-rate and high-energy-density lithium-ion battery anode containing 2D MoS2 nanowall and cellulose binder[J]. ACS Applied Materials & Interfaces, 2013, 5(4): 1240-1247. http://pubs.acs.org/doi/suppl/10.1021/am3022015/suppl_file/am3022015_si_001.pdf
[16] CHANG K, CHEN WX, MA L, et al. Graphene-like MoS2/amorphous carbon composites with high capacity and excellent stability as anode materials for lithium ion batteries[J]. Journal of Materials Chemistry, 2011, 21(17): 6251-6257. doi: 10.1039/c1jm10174a
[17] LEI ZD, XU LQ, JIAO YL, et al. Strong coupling of MoS2 nanosheets and nitrogen-doped graphene for high-performance pseudocapacitance lithium Storage[J]. Small, 2018, 14(25): 1704410. doi: 10.1002/smll.201704410
[18] YE W, WU FF, SHI NX, et al. Metal-Semiconductor phase twinned hierarchical MoS2 nanowires with expanded interlayers for sodium-ion batteries with ultralong cycle life[J]. Small, 2019, 16(3): 1906607. https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.201906607
[19] ZHU ZQ, TANG YX, LV ZS, et al. Fluoroethylene carbonate enabling robust LiF-rich solid electrolyte interphase to enhance the stability of MoS2 anode for lithium ion storage[J]. Angewandte Chemie, 2018, 57(14): 3656-3660. doi: 10.1002/anie.201712907
[20] BAI J, ZHAO BC, SHUAI L, et al. Construction of hierarchical V4C3-MXene/MoS2/C nanohybrids for high rate lithium-ion batteries[J]. Nanoscale, 2020, 12(2): 1144-1154. doi: 10.1039/C9NR07646H
[21] FANG YJ, LUAN DY, YE C, et al. Rationally designed three-layered Cu2S@Carbon@MoS2 hierarchical nanoboxes for efficient sodium storage[J]. Angewandte Chemie International Edition, 2020, 59(18): 7178-7183. doi: 10.1002/anie.201915917
[22] FUJIMOTO H, MABUCHI A, TOKUMITSU K, et al. Effect of crystallite size on the chemical compositions of the stage 1 alkali metal-graphite intercalation compounds[J]. Carbon, 1994, 32(2): 193-198. doi: 10.1016/0008-6223(94)90182-1
[23] ZAGHIB K, BROCHU F, GUERFI A, et al. Effect of particle size on lithium intercalation rates in natural graphite[J]. Journal of Power Sources, 2001, 103(1): 140-146. doi: 10.1016/S0378-7753(01)00853-9
-



 下载:
下载: