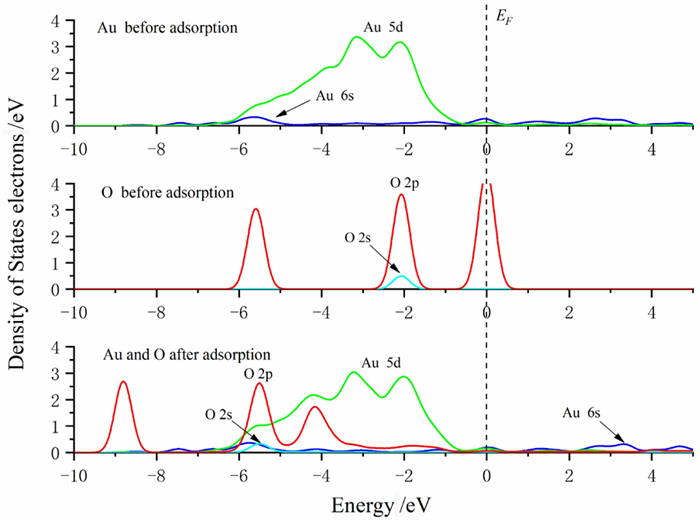
Density Functional Theory Study on Hydrophilic/hydrophobic Properties of Au (100) Surface
-
摘要:
采用密度泛函理论, 研究了单个、一层、多层(两层、三层)水分子与Au(100)表面的相互作用, 从原子层面上分析Au(100)表面的亲/疏水性质。同时结合分子动力学模拟, 分析水滴在金表面的润湿过程, 进而从介观层面上揭示金表面的亲/疏水性质。结果表明, 单个水分子在Au(100)表面是物理吸附。与单个水分子吸附构型相比, 一层水分子吸附构型中存在层内氢键作用, 多层水分子吸附构型中存在层内氢键和层间氢键作用。随着水分子层数的增多, 多层水分子吸附构型的内层水分子与Au(100)表面原子的平均作用距离呈现逐渐减小的趋势。水滴在金表面的分子动力学模拟结果表明, 有机污染会对金表面的润湿性产生较大影响, 导致金表面呈现一定的疏水性, 而清洁金表面则为亲水性。
Abstract:The interaction of single, one layer, multilayer (two layer, three layer) water molecules with Au(100) surface was studied Using density functional theory, and the hydrophilic/hydrophobic properties of Au(100) surface were analyzed at atomic level. In addition, the hydrophilic/hydrophobic properties of the gold surface were revealed from the mesoscopic level through analyzing the wetting process of water droplets on the gold surface by molecular dynamics simulation. The results show that individual water molecules are physically adsorbed on Au(100) surface. Compared with a single water molecule, hydrogen bond interaction exists between one and multiple layers of water molecules, and the adsorption energy of one or multiple layers of water molecules is obviously higher than that of a single water molecule. The adsorption energy increases with the increase of the number of water layers. Molecular dynamics simulation results of water droplets on gold surface show that organic pollution has a great influence on the wettability of gold surface, resulting in a certain hydrophobicity of gold surfacewhile clean gold surface is hydrophilic.
-
Key words:
- Au(100) /
- hydrophilic/hydrophobic /
- surface /
- density functional theory /
- wettability
-

-
表 1 单个水分子在Au(100)面的吸附能及作用距离
Table 1. Adsorption energy and action distance of a single water molecule on Au(100) surface
吸附构型 吸附位点 Eads/(kJ·mol-1) dO-Au/Å dH-Au/Å 单个水分子 顶位 -11.28 2.564 2.796 单个水分子 桥位 -7.14 3.086 2.809 单个水分子 穴位 -6.40 3.251 2.681 表 2 水分子的H、O原子和Au(100)表面的Au1原子吸附前后的Mulliken布居
Table 2. Mulliken populations of H and O atoms of water molecules and Au1 atoms before and after adsorption on Au (100) surface
元素 吸附状态 s p d f total Charge/e H1 吸附前 0.48 0.00 0.00 0.00 0.48 0.52 吸附后 0.56 0.00 0.00 0.00 0.56 0.44 H2 吸附前 0.48 0.00 0.00 0.00 0.48 0.52 吸附后 0.56 0.00 0.00 0.00 0.56 0.44 O 吸附前 1.90 5.14 0.00 0.00 7.04 -1.04 吸附后 1.87 4.96 0.00 0.00 6.83 -0.83 Au1 吸附前 2.91 6.41 9.68 14.00 33.00 -0.00 吸附后 2.82 6.48 9.66 14.00 32.96 0.04 表 3 一层、两层、三层水分子在Au(100)面的作用距离范围及平均作用距离
Table 3. Action distance range and average action distance of water molecules in the first, second and third layers on Au(100) surface
吸附构型 分层 内层水分子的氧与金原子作用距离
范围dO-Au/Å内层水分子的氧与金原子平均作用
距离dO-Au/Å一层水分子 一层 3.265~3.692 3.472 两层水分子 内层 2.534~3.668 3.203 三层水分子 内层 2.649~3.675 3.083 -
[1] 王燕东. 2009—2019年我国金矿资源勘查形势分析与对策[J]. 中国矿业, 2020, 29(11): 7-13. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGKA202011002.htm
WANG Y D. Analysis and suggestions of gold resources prospecting situation in China from 2009 to 2019[J]. China Mining Magazine, 2020, 29(11): 7-13. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGKA202011002.htm
[2] 任慧, 刘杰, 王勋, 等. 内蒙古某金矿阶段磨矿—阶段浮选试验研究[J]. 矿产保护与利用, 2021, 41(2): 106-111. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=50867c4c-d6e5-48f6-9dca-7fa984ca7a68
REN H, LIU J, WANG X, et al. Experimental study on stage grinding and stepwise flotation of a gold ore in inner mongolia[J]. Conservation and Utilization of Mineral Resources, 2021, 41(2): 106-111. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=50867c4c-d6e5-48f6-9dca-7fa984ca7a68
[3] 倪青青, 高志, 宋祖光. 提高河南某低品位金矿金回收率试验研究[J]. 金属矿山, 2020(9): 125-130. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS202009018.htm
NI Q Q, GAO Z, SONG Z G. Experiment on improving the gold recovery rate of a low-grade gold mine in Henan[J]. Metal Mine, 2020(9): 125-130. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS202009018.htm
[4] 陈桥, 杨洪英, 佟琳琳. 海南某金矿尼尔森重选-浮选试验[J]. 东北大学学报(自然科学版), 2020, 41(3): 413-417. https://www.cnki.com.cn/Article/CJFDTOTAL-DBDX202003020.htm
CHEN Q, YANG H Y, TONG L L. Processing a gold ore from Hainan province using knelson gravity concentration-flotation[J]. Journal of Northeastern University (Natural Science), 2020, 41(3): 413-417. https://www.cnki.com.cn/Article/CJFDTOTAL-DBDX202003020.htm
[5] R G J. The hydrophilic nature of gold and platinum[J]. Journal of Electroanalytical Chemistry & Interfacial Electrochemistry, 1977, 81(2): 285-290.
[6] 林斯, 马树江. 金矿浮选中某些化学状态[J]. 国外金属矿选矿, 1994, 31(9): 20-24. https://www.cnki.com.cn/Article/CJFDTOTAL-JSXK199409003.htm
LIN S, MA S J. Some chemical states in gold flotation[J]. Metallic Ore Dressing Abroad, 1994, 31(9): 20-24. https://www.cnki.com.cn/Article/CJFDTOTAL-JSXK199409003.htm
[7] WHITE M L. The wetting of gold surfaces by water [J]. Journal of Physical Chemistry, 2002, 68(10): 3083-3085.
[8] Erb, Robert A. Wettability of metals under continuous condensing conditions[J]. The Journal of Physical Chemistry, 1965, 69(4): 1306-1309. doi: 10.1021/j100888a035
[9] 江文峰, 陈建新, 吁松瑞, 等. 光响应"线性-超支化"超分子嵌段共聚物的制备及其对金表面的可逆亲疏水修饰[J]. 高分子学报, 2014(10): 1398-1407. doi: 10.11777/j.issn1000-3304.2014.14039
JIANG W F, CHEN J X, YU S R, et al. An amphiphilic linear-hyperbranched supramolecular block copolymer and its application in rendering phototuning wettability to gold surface[J]. Acta Polymerica Sinica, 2014(10): 1398-1407. doi: 10.11777/j.issn1000-3304.2014.14039
[10] FOWKES F M. Dispersion force contributions to surface and interfacial tensions, contact angles, and heats of immersion[J]. Advances in Chemistry, 1964, 43(6): 99-111.
[11] FOWKES F M. Attractive forces at interfaces[J]. Industrial & Engineering Chemistry, 1964, 56(12): 40-52.
[12] BARTELL F E, SMITH J T. Alteration of surface properties of gold and silver as indicated by contact angle measurements[J]. The Journal of Physical Chemistry, 1953, 57(2): 165-172. doi: 10.1021/j150503a008
[13] BEWIG K W, ZISMAN W A. The wetting of gold and platinum by water. [J]. Journal of Physical Chemistry, 1965, 69(12): 4238-4242. doi: 10.1021/j100782a029
[14] BERNETT M K, ZISMAN W A. Confirmation of spontaneous spreading by water on pure gold[J]. The Journal of Physical Chemistry, 1970, 74(11): 2309-2312. doi: 10.1021/j100705a012
[15] SCHRADER, MALCOLM E. Ultrahigh-vacuum techniques in the measurement of contact angles. Ⅱ. water on gold[J]. The Journal of Physical Chemistry, 1970, 74(11): 2313-2317. doi: 10.1021/j100705a013
[16] SMITH T. The hydrophilic nature of a clean gold surface[J]. Journal of Colloid & Interface Science, 1980, 75(1): 51-55.
[17] GARDNER J R. The hydrophilic nature of gold and platinum[J]. Journal of Electroanalytical Chemistry & Interfacial Electrochemistry, 1977, 81(2): 285-290.
[18] NEVES R S, MOTHEO A J, RUI P, et al. Modelling water adsorption on Au(210) surfaces. I. A force field for water-Au interactions by DFT[J]. Journal of Electroanalytical Chemistry, 2007, 609(2): 140-146.
[19] LIU R. Adsorption and dissociation of H2O on Au(111) surface: A DFT study[J]. Computational and Theoretical Chemistry, 2013, 1019: 141-145. doi: 10.1016/j.comptc.2013.07.009
[20] JIANG Z, LI M, YAN T, et al. Decomposition of H2O on clean and oxygen-covered Au (100) surface: A DFT study[J]. Applied Surface Science, 2014, 315: 16-21. doi: 10.1016/j.apsusc.2014.07.076
[21] VERTEGEL A A, SHUMSKY M G, SWITZER J A. Epitaxial electrodeposition of thallic oxide on single crystal gold[J]. Electrochimica Acta, 2000, 45(20): 3233-3239. doi: 10.1016/S0013-4686(00)00427-8
[22] SUH I K, OHTA H, WASEDA Y. High-temperature thermal expansion of six metallic elements measured by dilatation method and X-ray diffraction[J]. Journal of Materials Science, 1988, 23(2): 757-760. doi: 10.1007/BF01174717
[23] MARZARI N, VANDERBILT D, PAYNE M C. Ensemble density-functional theory for ab-initio molecular dynamics of metals and finite-temperature insulators[J]. Physical Review Letters, 1997, 79(7): 1337-1340. doi: 10.1103/PhysRevLett.79.1337
[24] XIAO C, XUE Q, LI X, et al. Inherent wettability of different rock surfaces at nanoscale: a theoretical study[J]. Applied Surface Science, 2018, 434: 73-81. doi: 10.1016/j.apsusc.2017.10.173
[25] GAO Y, ZHANG Y, YANG Y, et al. Molecular dynamics investigation of interfacial adhesion between oxidised bitumen and mineral surfaces[J]. Applied Surface Science, 2019, 479: 449-462. doi: 10.1016/j.apsusc.2019.02.121
[26] GUO J, ZHANG L, LIU S, et al. Effects of hydrophilic groups of nonionic surfactants on the wettability of lignite surface: Molecular dynamics simulation and experimental study[J]. Fuel, 2018, 231: 449-457. doi: 10.1016/j.fuel.2018.05.106
[27] YI H, JIA F, ZHAO Y, et al. Surface wettability of montmorillonite (001) surface as affected by surface charge and exchangeable cations: A molecular dynamic study[J]. Applied Surface Science, 2018, 459: 148-154. doi: 10.1016/j.apsusc.2018.07.216
[28] MICHAELIDES A. Density functional theory simulations of water-metal interfaces: waltzing waters, a novel 2D ice phase, and more[J]. Applied Physics A, 2006, 85(4): 415-425. doi: 10.1007/s00339-006-3695-9
[29] SCHNUR S, GRO A. Properties of metal-water interfaces studied from first principles[J]. New Journal of Physics, 2009, 11(12): 125003. doi: 10.1088/1367-2630/11/12/125003
-




 下载:
下载: