Synthesis of A New Hydroxamic Acid Collector and Its Collection Mechanism for Malachite
-
摘要:
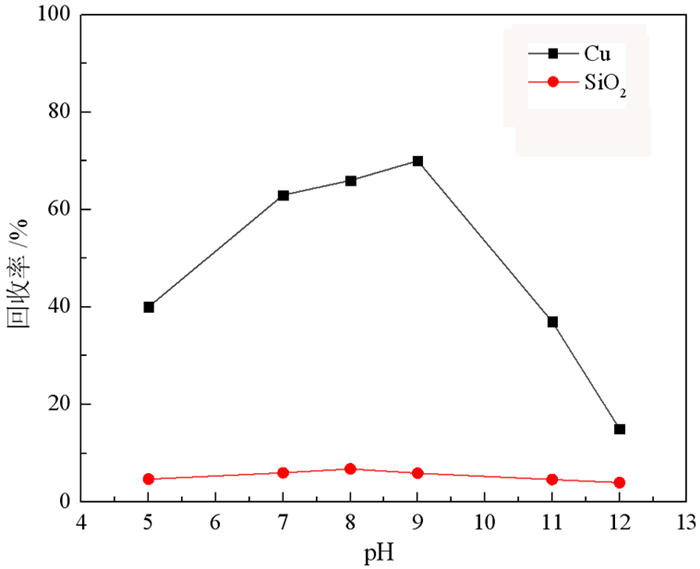
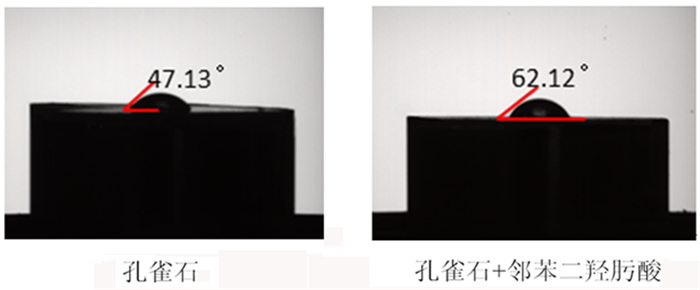
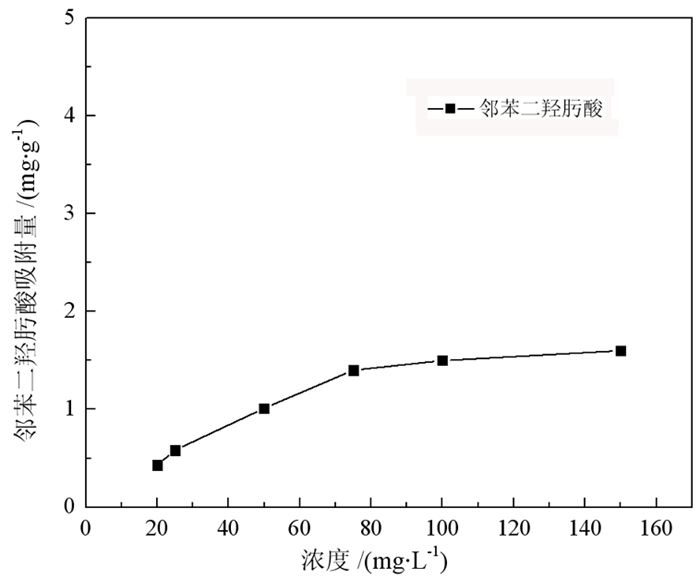
研究了一种新型螯合捕收剂邻苯二羟肟酸在孔雀石[Cu2CO3(OH)2]与石英(SiO2)浮选分离中的应用。通过微浮选试验, 评价了邻苯二羟肟酸对孔雀石和石英的浮选性能。结果表明, 邻苯二羟肟酸对孔雀石有较强的吸附和选择性, 能有效分离孔雀石和石英。以邻苯二羟肟酸为捕收剂, 在pH为9、药剂用量80 mg/L的条件下对人工混合矿物具有良好的分离效果, 孔雀石回收率70%, 石英回收率5%。通过接触角、SEM-EDS、Zeta电位、吸附量、傅里叶变换红外光谱(FT-IR)和X射线光电子能谱(XPS)分析研究了吸附机理, 结果表明邻苯二羟肟酸的与孔雀石表面的Cu2+离子发生强烈的化学吸附, 处理后孔雀石疏水性大大提高, 选择性较好, 可有效分离孔雀石与脉石矿物。
Abstract:The application of phthalic acid, a new chelating collector, in the flotation separation of malachite (Cu2CO3(OH)2) and quartz (SiO2) was studied. The flotation performance of phthalic acid on malachite and quartz was evaluated by micro-flotation test. The results show that phthalic acid has strong adsorption and selectivity for malachite and can effectively separate malachite and quartz. Using phthalic acid as collector, the separation effect of artificial mixed minerals was good at pH 9 and dosage of 80 mg/L. And the recovery of malachite and quartz was 70% and 5%, respectively. The adsorption mechanism was studied by contact angle, SEM-EDS, Zeta potential, adsorption capacity, Fourier Transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS). The results showed that the strong chemical adsorption between phthalic acid and Cu2+ ions on malachite surface occurred. After treatment, the hydrophobicity of malachite was greatly improved and the selectivity was good. It can effectively separate malachite and gangue minerals.
-
Key words:
- malachite /
- quartz /
- hydroxamic acid /
- flotation /
- flotation mechanism /
- copper
-

-
表 1 孔雀石纯矿物X射线荧光光谱分析
Table 1. X-ray fluorescence spectrum analysis of pure malachite
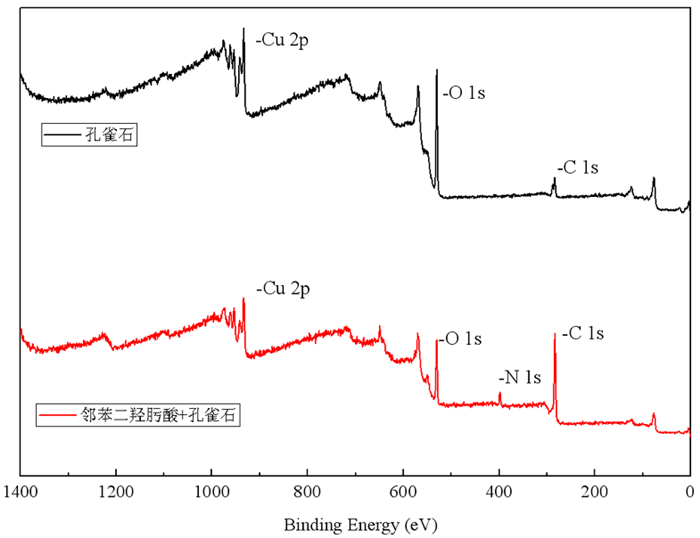
/% 元素 Cu Al Zn P S Si Fe Mn Cl 其他 含量 55.83 0.07 0.05 0.04 0.003 0.06 0.01 0.02 0.01 43.91 表 2 邻苯二羟肟酸处理前后孔雀石的元素含量
Table 2. Atomic concentrations of elements before and after treatment of Malachite with o-dihydroxamic acid
Species Atomic concentration/% C 1s N 1s O 1s Cu 2p 孔雀石 33.37 0.00 52.67 13.96 孔雀石+邻苯二羟肟酸 36.33 2.13 50.78 10.76 -
[1] SUN Q Y, YIN W Z, LI D, et al. Improving the sulfidation flotation of fine cuprite by hydrophobic flocculation pretreatment[J]. International Journal of Minerals, Metallurgy, and Materials, 2018, 25: 1256-1262. doi: 10.1007/s12613-018-1678-4
[2] BAI X, WEN S, FENG Q, et al. Utilization of high-gradient magnetic separation-secondary grinding-leaching to improve the copper recovery from refractory copper oxide ores[J]. Minerals Engineering, 2019, 136: 77-80. doi: 10.1016/j.mineng.2019.03.009
[3] CHEN X M, PENG Y J, et al. The separation of chalcopyrite and chalcocite from pyrite in cleaner flotation after regrinding[J]. Minerals Engineering, 2014, 58: 64-72. doi: 10.1016/j.mineng.2014.01.010
[4] LIU R Z, LIU D W, et al. Sulfidization mechanism in malachite flotation: A heterogeneous solid-liquid reaction that yields CuxSy phases grown on malachite[J]. Minerals Engineering, 2020, 154: 106420. doi: 10.1016/j.mineng.2020.106420
[5] YIN W Z, SUN Q Y, et al. Mechanism and application on sulphidizing flotation of copper oxide with combined collectors[J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 178-185. doi: 10.1016/S1003-6326(18)64926-X
[6] MARION C, JORDENS A, LI R H, et al. An evaluation of hydroxamate collectors for malachite flotation[J]. Separation and Purification Technology, 2017, 183: 258-269. doi: 10.1016/j.seppur.2017.02.056
[7] DENG J S, WEN S M, QIONG Y, et al. Leaching of malachite using 5-sulfosalicylic acid[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 20-27. doi: 10.1016/j.jtice.2016.11.013
[8] ZHOING C, FENG B, WANG H, et al. The depression behavior and mechanism of tragacanth gum on chalcopyrite during Cu-Mo flotation separation[J]. Advanced Powder Technology, 2021, 32: 2712-2719. doi: 10.1016/j.apt.2021.05.032
[9] CHEN Y, FENG B, GUO Y, et al. The role of oxidizer in the flotation separation of chalcopyrite and galena using sodium lignosulfonate as a depressant[J]. Minerals Engineering, 2021, 172: 107160. doi: 10.1016/j.mineng.2021.107160
[10] ZHANG X R, LU L, ZHU Y G, et al. Research on the separation of malachite from quartz with S-carboxymethyl-O, O-dibutyl dithiophosphate chelating collector and its insights into flotation mechanism[J]. Powder Technology, 2020, 366: 130-136. doi: 10.1016/j.powtec.2020.02.071
[11] CECILE J L, CRUZ M I, BARBERY G, et al. Infrared spectral study of species formed on malachite surface by adsorption from aqueous salicylaldoxime solution[J]. Journal of Colloid and Interface Science. 1981, 80: 589-597. doi: 10.1016/0021-9797(81)90215-0
[12] LI F, ZHOU X T, LIN R X. Flotation performance and adsorption mechanism of novel1-(2-hydroxyphenyl)hex-2-en-1-one oxime flotation collector tomalachite[J]. Transactions of Nonferrous Metals Society of China. 2020, 30: 2792-2801. doi: 10.1016/S1003-6326(20)65421-8
[13] LI Z L, RAO F, SONG S X, et al. Effects of commonions on adsorption and flotation of malachite with salicylaldoxime[J]. Colloid Surface A. 2019, 577: 421-428. doi: 10.1016/j.colsurfa.2019.06.004
[14] LI L Q, ZHAO J H, XIAO Y Y, et al. Flotation performance and adsorption mechanism of malachite with tert-butylsalicylaldoxime[J]. Separation and Purification Technology, 2019, 210: 843-849. doi: 10.1016/j.seppur.2018.08.073
[15] HUANG K, CAO Z, WANG S, et al. Flotation performance and adsorption mechanism of styryl phosphonate mono-iso-octyl ester to malachite[J]. Colloids and Surfaces A Physicochemical and Engineering Aspects, 2019, 579: 123698. doi: 10.1016/j.colsurfa.2019.123698
[16] FENG, Q C, ZHAO W J, et al. Ammonia modification for enhancing adsorption of sulfide species onto malachite surfaces and implications for flotation[J]. Journal of Alloys and Compounds: An Interdisciplinary Journal of Materials Science and Solid-state Chemistry and Physics, 2018, 744: 301-309.
[17] 杨绵延, 马英强, 于岩, 等. 孔雀石分段硫化的浮选行为及机理研究[J]. 矿产保护与利用, 2021, 41(2): 80-88. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=b73512f0-70d6-4062-80b6-c0880224398c
YANG M Y, MA Y Q, YU Y, et al. Malachite segmented flotation behavior of sulfide and mechanism research[J]. Conservation and Utilization of Mineral Resources, 2021, 41(2): 80-88. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=b73512f0-70d6-4062-80b6-c0880224398c
[18] WANG H, WEN S, HAN G, et al. Modification of malachite surfaces with lead ions and its contribution to the sulfidization flotation[J]. Applied Surface Science, 2021, 550: 149350. doi: 10.1016/j.apsusc.2021.149350
[19] PARK K, PARK S, CHOI J, et al. Influence of excess sulfide ions on the malachite-bubble interaction in the presence of thiol-collector[J]. Separation & Purification Technology, 2016, 168: 1-7.
[20] LIU C, SONG S, LI H, et al. Sulfidization flotation performance of malachite in the presence of calcite[J]. Minerals Engineering, 2019, 132: 293-296. doi: 10.1016/j.mineng.2018.11.051
[21] CHOI J, CHOI S, PARK K, et al. Flotation behaviour of malachite in mono- and di valent salt solutions using sodium oleate as a collector[J]. International Journal of Mineral Processing, 2016, 146: 38-45. doi: 10.1016/j.minpro.2015.11.011
[22] FAN H, OIN J, LIU G, et al. Investigation into the flotation of malachite calcite and quartz with three phosphate surfactants[J]. Journal of Materials Research and Technology, 2019, 8: 5140-5148. doi: 10.1016/j.jmrt.2019.08.037
[23] LI F X, ZHOU X T, ZHAO G. A novel decylsalicylhydroxamic acid flotation collector: Its synthesis and flotation separation of malachite against quartz[J]. PowderTechnology. 2020, 374: 522-526.
[24] 黄凌云, 孙鑫, 杨思原, 等. 氧化铜矿浮选捕收剂研究进展[J]. 矿产保护与利用, 2020, 40(2): 88-92. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=2dc30c77-219a-427a-91e2-898b98154727
HUANG L Y, SUN X, YANG S Y, et al. Application and Research Progress of Flotation Collectors for Copper Oxide Ore[J]. Conservation and Utilization of Mineral Resources, 2020, 40(2): 88-92. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=2dc30c77-219a-427a-91e2-898b98154727
[25] 陈代雄, 严宇扬, 肖骏, 等. 苯甲羟肟酸和丁基黄药协同浮选氧化铜矿石试验[J]. 现代矿业, 2015(8): 4. https://www.cnki.com.cn/Article/CJFDTOTAL-KYKB201508025.htm
CHEN D X, YAN Y Y, XIAO J, et al. Copper Oxide Ore Flotation Test by Synergy of Benzohydroxamic Acid and Butyl Xanthate[J]. Modern Mining, 2015(8): 4. https://www.cnki.com.cn/Article/CJFDTOTAL-KYKB201508025.htm
[26] 孟庆波, 徐晓萍, 高玉德, 等. 辛基羟肟酸钠和丁基黄药混合使用对孔雀石浮选行为的影响[J]. 金属矿山, 2018(6): 5. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS201806015.htm
MENG Q B, XU X P, GAO Y D, et al. Influence of Combined Use of Sodium Octyl Hydroxamate Acid and Butyl Xanthate on Flotation Behavior of Malachite[J]. METAL MINE, 2018(6): 5. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS201806015.htm
[27] LU Y, WU K, WANG S, et al. Structural modification of hydroxamic acid collectors to enhance the flotation performance of malachite and associated mechanism[J]. Journal of Molecular Liquids, 2021, 344: 117959. doi: 10.1016/j.molliq.2021.117959
[28] YU X, ZHANG R, ZENG Y, et al. The effect and mechanism of cinnamic hydroxamic acid as a collector in flotation separation of malachite and calcite[J]. Minerals Engineering, 2021, 164: 106847. doi: 10.1016/j.mineng.2021.106847
[29] HUANG K, CAO Z, WANG S, et al. Flotation performance and adsorption mechanism of styryl phosphonate mono-iso-octyl ester to malachite[J]. Colloids and Surfaces A Physicochemical and Engineering Aspects, 2019, 579: 123698. doi: 10.1016/j.colsurfa.2019.123698
[30] CHOI J, CHOI S Q, PARK K, e tal. Flotation behaviour of malachite in mono- and di-valent salt solutions using sodium oleate as a collector[J]. International Journal of Mineral Processing, 2016, 146: 38-45. doi: 10.1016/j.minpro.2015.11.011
[31] LI L Q, ZHAO J H, XIAO Y Y, et al. Flotation performance and adsorption mechanism of malachite with tert-butylsalicylaldoxime[J]. Separation and Purification Technology, 2019, 210: 843-849.
[32] 王浩林. 新型羟肟酸捕收剂制备及其对氟碳铈矿浮选特性与机理研究[D]. 赣州: 江西理工大学, 2019: 1.
[33] WANG H, WEN S, HAN G, et al. Modification of malachite surfaces with lead ions and its contribution to the sulfidization flotation[J]. Applied Surface Science, 2021, 550: 149350.
[34] WANG H, WEN S, HAN G, et al. Adsorption characteristics of Pb (Ⅱ)species on the sulfidized malachite surface and its response to flotation[J]. Separation and Purification Technology, 2021, 264: 118-126.
[35] LENORMAND J, SALMAN T, YOON R H. Hydroxamate flotation of malachite[J]. Canadian Metallurgical Quarterly, 1979, 18: 125-129.
[36] YU X, ZANG R, ZENG Y, et al. The effect and mechanism of cinnamichydroxamic acid as a collector in flotation separation of malachite and calcite[J]. Minerals Engineering, 2021, 164: 106847.
[37] QIAN Z A, YW B, QF A, et al. Identification of sulfidization products formed on azurite surfaces and its correlations with xanthate adsorption and flotation - ScienceDirect[J]. Applied Surface Science, 2020, 511: 145594.
[38] FENG Q, ZHAO W, WEN S, et al. Copper sulfide species formed on malachite surfaces in relation to flotation[J]. Journal of Industrial & Engineering Chemistry, 2017, 48: 125-132.
-




 下载:
下载: