Effect and Mechanism of Depressant Amino Trimethylene Phosphonic Acid on Flotation Separation of Magnesite and Dolomite
-
摘要:
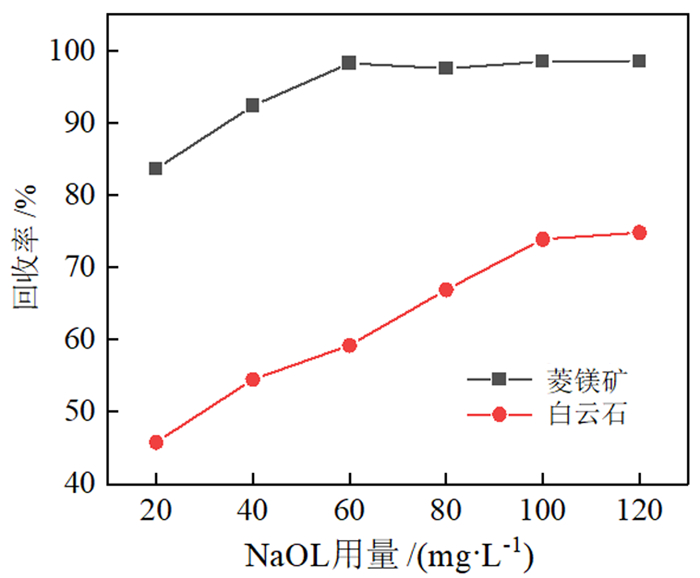
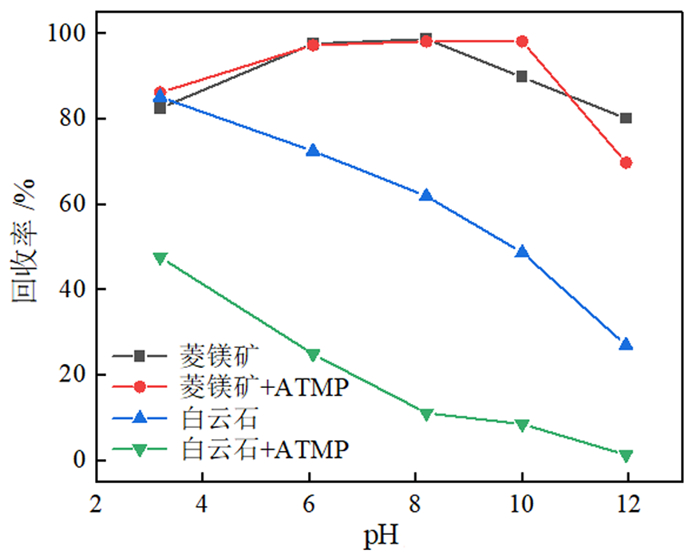
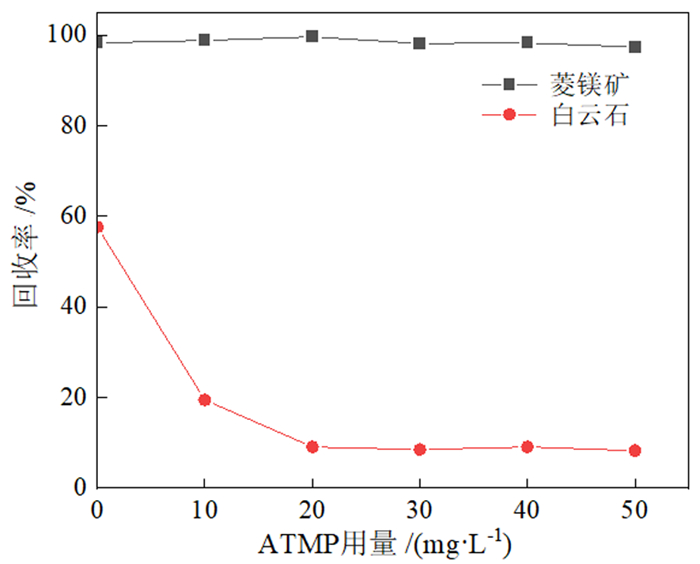
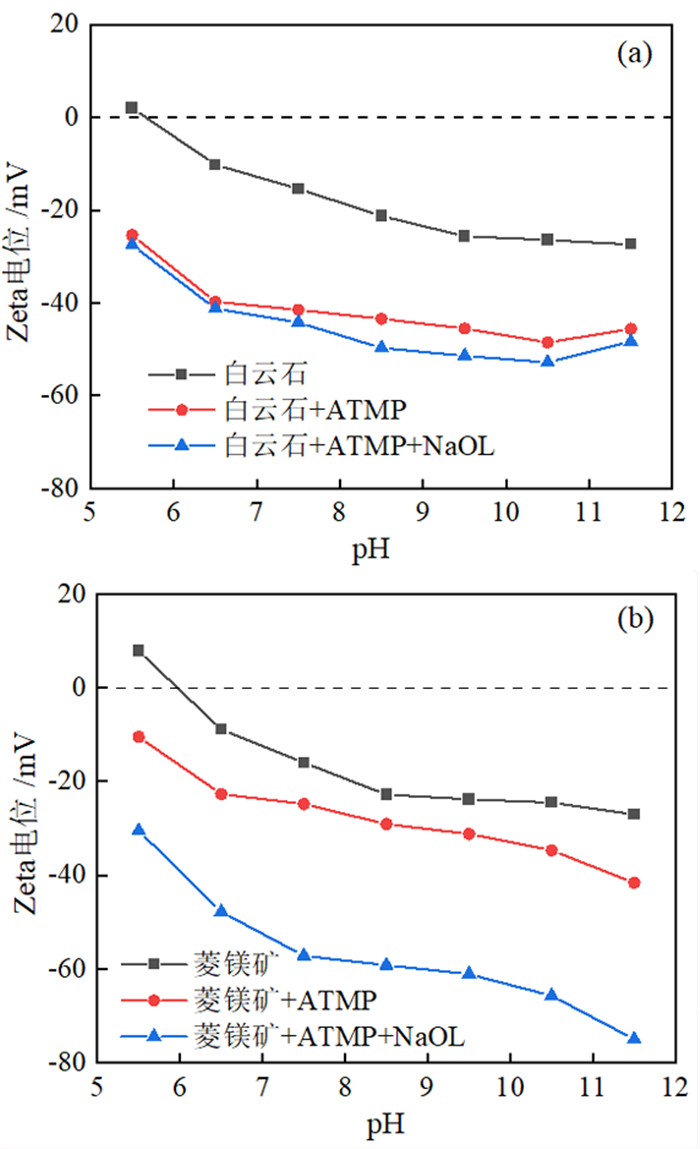
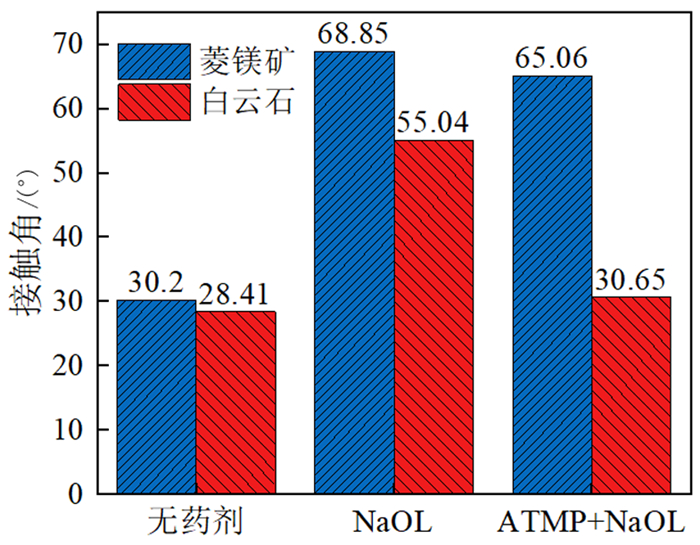
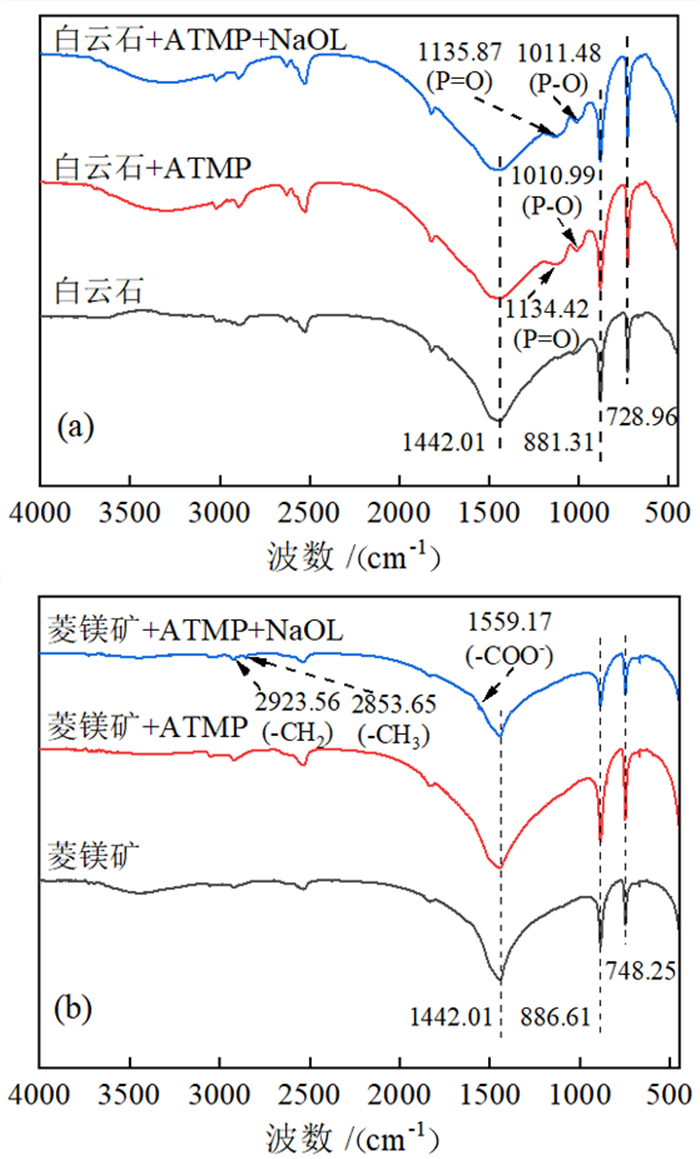
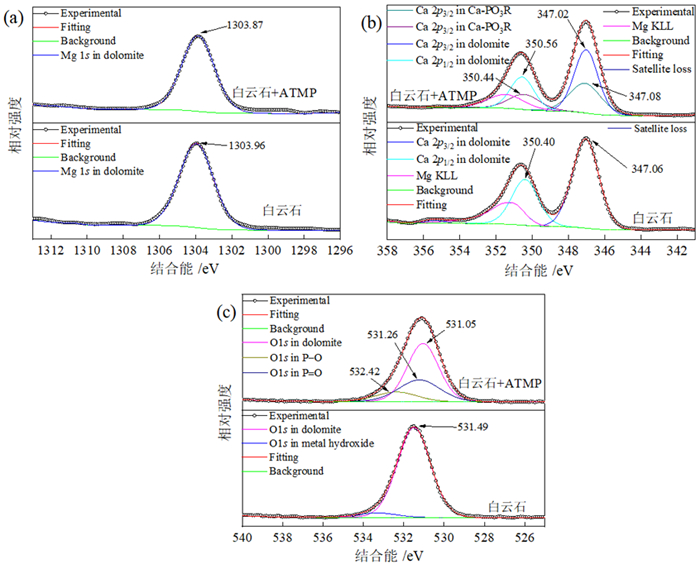
如何利用高效分离抑制剂实现白云石与菱镁矿的有效分离是含钙型菱镁矿矿石加工利用的研究重点。本文在油酸钠(NaOL)体系下,以氨基三亚甲基膦酸(ATMP)为抑制剂,通过单矿物浮选和人工混合矿浮选试验,考察其对菱镁矿和白云石浮选行为的影响。在此基础上,利用Zeta电位、接触角、红外光谱和X射线光电子能谱等手段揭示了ATMP在两种矿物表面的作用机制。结果表明,针对菱镁矿与白云石质量比4 : 1的人工混合矿,在pH=10、NaOL用量为60 mg/L、ATMP用量为20 mg/L时,可获得MgO品位43.98%、CaO品位3.30%、MgO回收率91.18%的菱镁矿精矿,分选效率达91.18%。ATMP可强烈抑制白云石,而对菱镁矿的浮选几乎没有影响。ATMP通过与白云石表面的Ca位点强烈作用,占据白云石表面活性位点,并通过静电排斥作用阻碍NaOL在白云石表面吸附,进而扩大了菱镁矿和白云石表面疏水性能的差异,实现了对白云石的选择性抑制。
Abstract:The highly effective depressant of dolomite from magnesite has been the focus in the field of mineral processing. Using sodium oleate (NaOL) as collector and amino trimethylene phosphonic acid (ATMP) as the depressant, the effects of these reagents on the flotation behavior of magnesite and dolomite were investigated through single and artificial mixed ore flotation tests. Furthermore, the mechanism of ATMP on the surfaces of both minerals was revealed using zeta potential, contact angle measurements, FTIR and XPS. Form the artificial mixed ore with magnesite and dolomite in the mass ratio of 4 : 1, the flotation indexes with MgO grade of 43.98%, CaO grade of 3.30%, recovery of 91.18% and separation efficiency of 91.18% could be obtained at approximately pH 10 with a reagent scheme of 20 mg/L ATMP and 60 mg/L NaOL. The results indicated that ATMP displayed an excellent depression effect on the dolomite flotation, whereas it rarely had an influence on magnesite. ATMP occupied a large number of active sites on dolomite surface through strongly interacting with the calcium sites, hindering the adsorption of NaOL on dolomite surface via electrostatic repulsion. In summary, the differential adsorption of the ATMP depressant onto magnesite and dolomite magnified the difference in hydrophobicity between them, which realizing the selective inhibition of dolomite.
-
Key words:
- magnesite /
- dolomite /
- depressant /
- amino trimethylene phosphonic acid /
- mechanism
-

-
表 1 菱镁矿和白云石样品的多元素化学分析
Table 1. Chemical compositions of magnesite and dolomite
Minerals Compositions MgO CaO SiO2 Al2O3 Total Fe Magnesite 46.50 0.51 0.35 0.09 0.28 Dolomite 20.91 30.70 0.34 0.33 0.07 表 2 不同药剂制度下菱镁矿和白云石人工混合矿浮选结果
Table 2. Flotation results of mixed magnesite and dolomite treated with different reagents
Reagents Products Yield/% Grade/% Recovery/% E/% MgO CaO MgO CaO NaOL: 60 mg/L Concentrate 90.77 41.89 5.43 91.76 75.16 81.32 Tailing 9.23 36.36 17.55 8.10 24.69 Feed 100.00 41.38 6.55 99.85 99.85 ATMP: 20 mg/L
NaOL: 60 mg/LConcentrate 85.79 43.98 3.30 91.18 43.23 91.18 Tailing 14.21 25.69 26.15 8.82 56.77 Feed 100.00 41.38 6.55 100.00 100.00 -
[1] PRASAD S V S, PRASAD S B, VERMA K, et al. The role and significance of Magnesium in modern day research-A review[J]. Journal of Magnesium and Alloys, 2022, 10: 1-61. doi: 10.1016/j.jma.2021.05.012
[2] 乌志明, 马培华. 镁、镁资源与镁质材料概述[J]. 盐湖研究, 2007, 15(4): 65-72. doi: 10.3969/j.issn.1008-858X.2007.04.012
WU Z M, MA P H. Summary of magnesium, magnesium resources and magnesium materials[J]. Journal of Salt Lake Research, 2007, 15(4): 65-72. doi: 10.3969/j.issn.1008-858X.2007.04.012
[3] WONYEN D G, KROMAH V, GIBSON B, et al. A review of flotation separation of Mg carbonates (dolomite and magnesite)[J]. Minerals, 2018, 8(8): 354-354. doi: 10.3390/min8080354
[4] LIU W B, PENG X Y, LIU W G, et al. Effect mechanism of the iso-propanol substituent on amine collectors in the flotation of quartz and magnesite[J]. Powder Technology, 2020, 360: 1117-1125. doi: 10.1016/j.powtec.2019.10.060
[5] LIU A P, NI W, WU W. Mechanism of separating pyrite and dolomite by flotation[J]. Journal of University of Science and Technology Beijing, Mineral, Metallurgy, Material, 2007, 14(4): 291-296. doi: 10.1016/S1005-8850(07)60057-7
[6] GENCE N. Wetting behavior of magnesite and dolomite surfaces[J]. Applied Surface Science, 2006, 252(10): 3744-3750. doi: 10.1016/j.apsusc.2005.05.053
[7] PELEKA E N, GALLIOS G P, MATIS K A. A perspective on flotation: A review[J]. Journal of Chemical Technology & Biotechnology, 2018, 93(3): 615-623.
[8] HAN C, ZHANG H, TAN R, et al. Effects of monohydric alcohols of varying chain lengths and isomeric structures on magnesite and dolomite flotation by dodecylamine[J]. Powder Technology, 2020, 374: 233-240. doi: 10.1016/j.powtec.2020.07.048
[9] TANG Y, YIN W Z, KELEBEK S. Molecular dynamics simulation of magnesite and dolomite in relation to flotation with cetyl phosphate[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 610: 125928. doi: 10.1016/j.colsurfa.2020.125928
[10] LUO N, WEI D Z, SHEN Y B, et al. Elimination of the adverse effect of calcium ion on the flotation separation of magnesite from dolomite[J]. Minerals, 2017, 7(8): 150. doi: 10.3390/min7080150
[11] YAO J, SUN H R, HAN F, et al. Enhancing selectivity of modifier on magnesite and dolomite surfaces by pH control[J]. Powder Technology, 2020, 362: 698-706. doi: 10.1016/j.powtec.2019.12.040
[12] CHEN G L, TAO D. Reverse flotation of magnesite by dodecyl phosphate from dolomite in the presence of sodium silicate[J]. Separation science and technology, 2005, 39(2): 377-390. doi: 10.1081/SS-120027564
[13] SHI Q, FENG Q M, ZHANG G F, et al. A novel method to improve depressants actions on calcite flotation[J]. Minerals Engineering, 2014, 55: 186-189. doi: 10.1016/j.mineng.2013.10.010
[14] TIAN M J, GAO Z Y, HAN H S, et al. Improved flotation separation of cassiterite from calcite using a mixture of lead (Ⅱ) ion/benzohydroxamic acid as collector and carboxymethyl cellulose as depressant[J]. Minerals Engineering, 2017, 113: 68-70. doi: 10.1016/j.mineng.2017.08.010
[15] CHEN W, FENG Q M, ZHANG G F, et al. Selective flotation of scheelite from calcite using calcium lignosulphonate as depressant[J]. Minerals Engineering, 2018, 119: 73-75. doi: 10.1016/j.mineng.2018.01.015
[16] 王霞, 白媛丽, 思玉琥, 等. 氨基三甲叉膦酸的合成及其缓蚀阻垢性能[J]. 腐蚀与防护, 2012, 33(5): 404-410. https://www.cnki.com.cn/Article/CJFDTOTAL-FSYF201205014.htm
WANG X, BAI Y L, SI Y H, et al. Synthesis and performance of amino trimethylene phosphonic acid[J]. Corrosion & Protection, 2012, 33(5): 404-410. https://www.cnki.com.cn/Article/CJFDTOTAL-FSYF201205014.htm
[17] LIU C, AI G H, SONG S X. The effect of amino trimethylene phosphonic acid on the flotation separation of pentlandite from lizardite[J]. Powder Technology, 2018, 336: 527-532. doi: 10.1016/j.powtec.2018.06.030
[18] CHEN Y F, TANG X K. Selective flotation separation of smithsonite from calcite by application of amino trimethylene phosphonic acid as depressant[J]. Applied Surface Science, 2020, 512: 145663. doi: 10.1016/j.apsusc.2020.145663
[19] TANTAYAKOM V, FOGLER H S, DE MORAES F F, et al. Study of Ca-ATMP precipitation in the presence of magnesium Ion[J]. Langmuir, 2004, 20(6): 2220-2226. doi: 10.1021/la0358318
[20] LIU W B, LIU W G, ZHAO B, et al. Novel insights into the adsorption mechanism of the isopropanol amine collector on magnesite ore: a combined experimental and theoretical computational study[J]. Powder Technology, 2019, 343: 366-374. doi: 10.1016/j.powtec.2018.11.063
[21] YANG B, WANG D H, CAO S H, et al. Selective adsorption of a high-performance depressant onto dolomite causing effective flotation separation of magnesite from dolomite[J]. Journal of Colloid and Interface Science, 2020, 578: 290-303. doi: 10.1016/j.jcis.2020.05.100
[22] XU L H, ZHANG S L, GUO S Y, et al. ATMP derived cobalt-metaphosphate complex as highly active catalyst for oxygen reduction reaction[J]. Journal of Catalysis, 2020, 387: 129-137. doi: 10.1016/j.jcat.2020.04.014
[23] YIN W Z, SUN H R, TANG Y, et al. Effect of pulp temperature on separation of magnesite from dolomite in sodium oleate flotation system[J]. Physicochemical Problems of Mineral Processing, 2019, 55: 1049-1058.
[24] WANG J J, LI W H, ZHOU Z H, et al. 1-Hydroxyethylidene-1, 1-diphosphonic acid used as pH-dependent switch to depress and activate fluorite flotation I: depressing behavior and mechanism[J]. Chemical Engineering Science, 2020, 214: 115369. doi: 10.1016/j.ces.2019.115369
[25] STRANICK M A, ROOT M J. Influence of strontium on monofluorophosphate uptake by hydroxyapatite XPS characterization of the hydroxyapatite surface[J]. Colloids and surfaces, 1991, 55: 137-147. doi: 10.1016/0166-6622(91)80088-6
[26] GÖTTLICHER S, VEGAS A. Electron-density distribution in magnesite (MgCO3)[J]. Acta Crystallographica Section B: Structural Science, 1988, 44(4): 362-367. doi: 10.1107/S0108768188002332
[27] LAÇIN O, DÖNMEZ B, DEMIR F. Dissolution kinetics of natural magnesite in acetic acid solutions[J]. International Journal of Mineral Processing, 2005, 75(1/2): 91-99.
[28] CHEN G L, TAO D. Effect of solution chemistry on flotability of magnesite and dolomite[J]. International Journal of Mineral Processing, 2004, 74(1/2/3/4): 343-357.
[29] 孙文瀚, 代淑娟, 罗娜, 等. 基于矿石溶解性差异的菱镁矿酸浸脱钙[J]. 中国有色金属学报, 2019, 29(8): 1733-1739. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201908018.htm
SUN W H, DAI S J, LUO N, et al. Decalcification leaching test of magnesite based on solubleness difference of minerals[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(8): 1733-1739. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201908018.htm
[30] GLEDHILL W E, FEIJTEL T C J. Environmental properties and safety assessment of organic phosphonates used for detergent and water treatment applications[M]. Berlin: Springer, 1992: 261-285.
-



 下载:
下载: