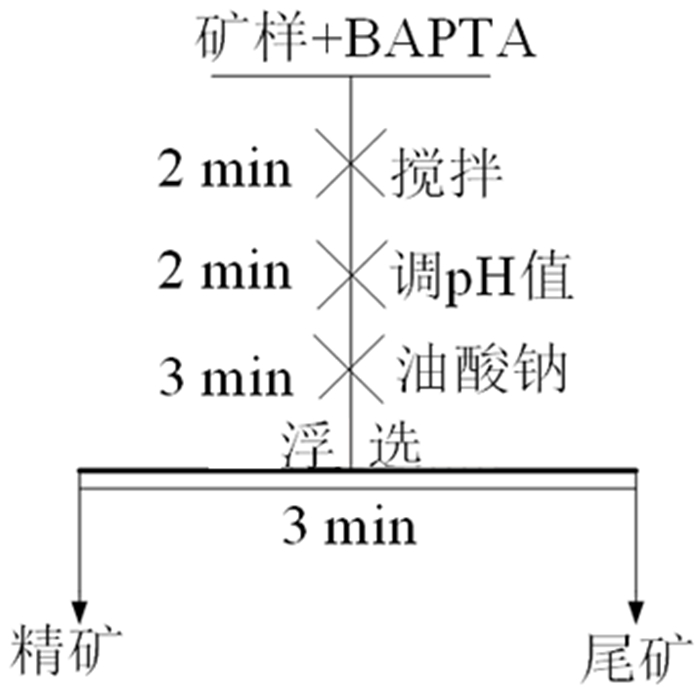
Mechanism of Chelating Depressant BAPTA in Flotation Separation of Magnesite and Calcite
-
摘要:
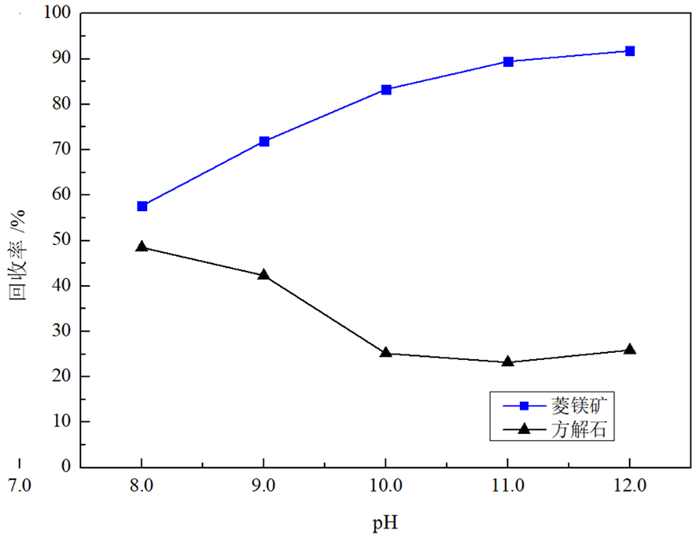
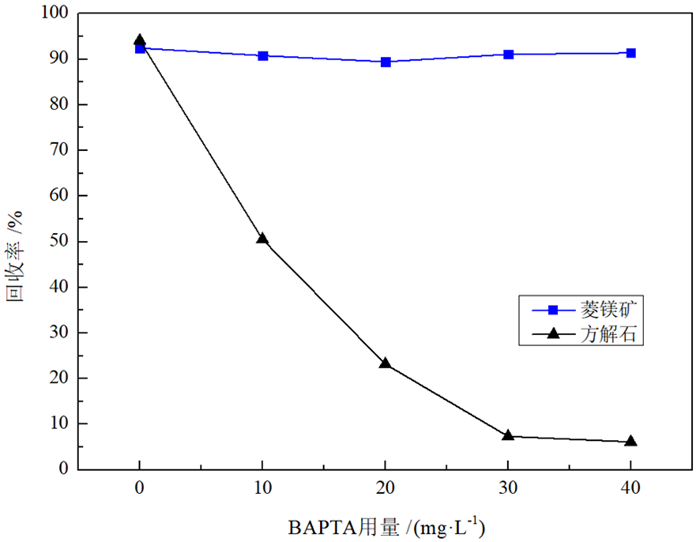
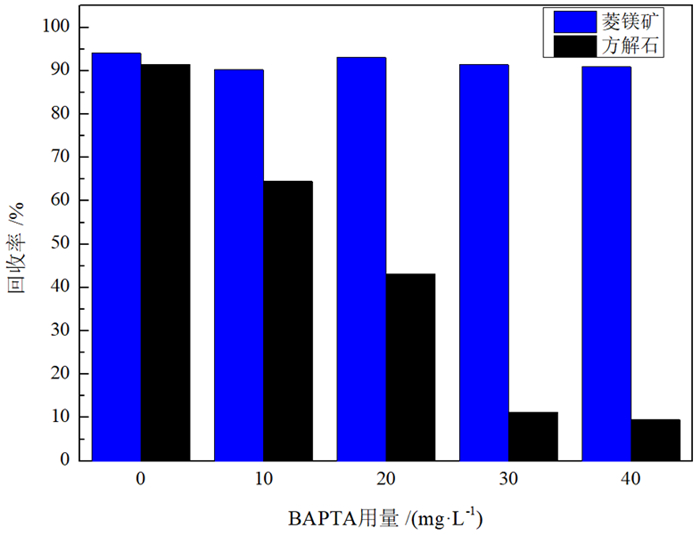
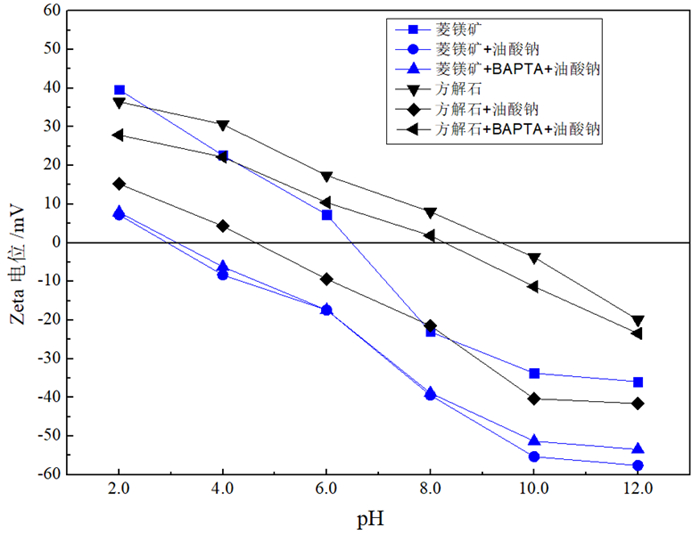
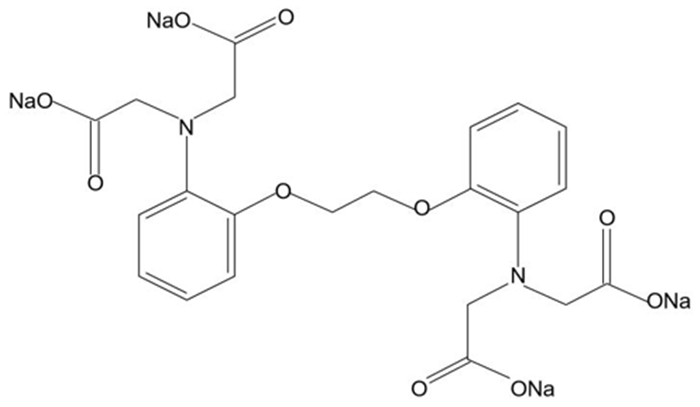
由于菱镁矿和方解石具有相似的晶体结构和化学性质,故通过浮选较难实现二者的有效分离。BAPTA作为一种Ca-选择性螯合剂,被用以改善菱镁矿与方解石的浮选分离。浮选试验结果表明,在油酸钠体系下,BAPTA能选择性地抑制方解石上浮,且在矿浆pH值11.0、BAPTA用量30 mg/L和油酸钠用量100 mg/L的条件下,可较好实现菱镁矿(回收率91.06%)与方解石(回收率7.37%)的浮选分离。利用Zeta电位、FTIR和XPS等检测方法研究了BAPTA的选择抑制机理,结果表明,BAPTA能选择性地与方解石表面的Ca发生反应,吸附并罩盖在方解石表面,阻止油酸钠在方解石表面吸附,消除油酸钠给方解石带来的零电点负移的影响,但BAPTA对菱镁矿表面的Mg作用较小,故对菱镁矿吸附油酸钠的影响较小。
Abstract:It is difficult to achieve the effective separation of magnesite from calcite because of their similar chemical and crystal properties. As a Ca selective chelator, BAPTA was used to improve the flotation separation of magnesite and calcite. The flotation test results showed that BAPTA could selectively inhibit the upward flotation of calcite in the sodium oleate system, and the flotation recovery difference between magnesite (recovery: 91.06%) and calcite (recovery: 7.37%) was large at pH 11.0, BAPTA dosage of 30 mg/L and sodium oleate dosage of 100 mg/L, which revealed that the flotation separation of magnesite and calcite could be realized. The selective depression mechanism of BAPTA was studied by Zeta potential, FTIR and XPS. The results indicated that BAPTA could selectively react with Ca on the surface of calcite, adsorb and cover the surface of calcite, prevent the adsorption of sodium oleate onto calcite surface, and eliminate the negative shift of zero electric point caused by sodium oleate on calcite. However, BAPTA had a little influence on Mg on the surface of magnesite, so it has a little effect on the adsorption of sodium oleate on magnesite.
-
Key words:
- magnesite /
- calcite /
- BAPTA /
- selective depression /
- flotation separation
-

-
表 1 单矿物化学组分分析
Table 1. Chemical analysis of pure minerals
/% 矿物 MgO CaO SiO2 Al2O3 TFe 纯度 菱镁矿 45.68 1.20 0.80 0.055 0.15 95.93 方解石 0.31 55.55 < 0.05 0.22 0.019 99.15 表 2 试验主要试验试剂
Table 2. Main reagents list of test
试剂名称 化学式 纯度 用途 生产商 油酸钠 C18H33O2Na 分析纯 捕收剂 MACKLIN., Shanghai BAPTA C22H20N2Na4O10 化学纯 抑制剂 MACKLIN., Shanghai 盐酸 HCl 分析纯 pH调整剂 MACKLIN., Shanghai 氢氧化钠 NaOH 分析纯 pH调整剂 MACKLIN., Shanghai 溴化钾 KBr 光谱纯 红外基底 MACKLIN., Shanghai 氯化钾 KCl 分析纯 电解质 MACKLIN., Shanghai 表 3 BAPTA作用前后矿物表面元素含量及结合能分析
Table 3. Analysis of mineral surface element content and binding energy before and after BAPTA action
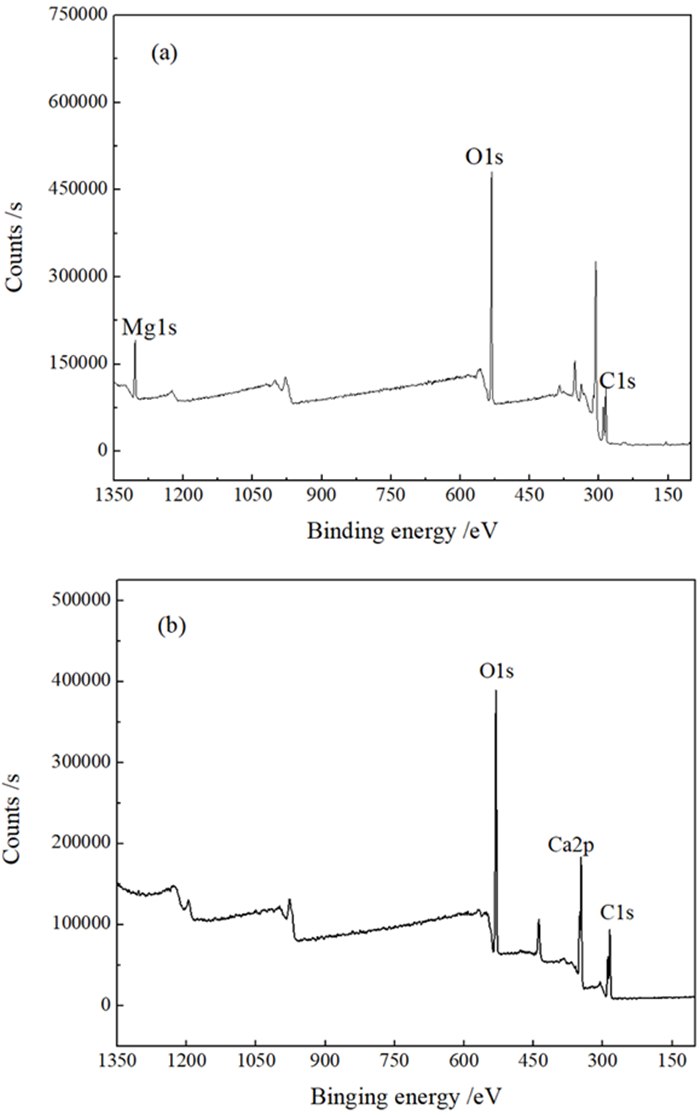
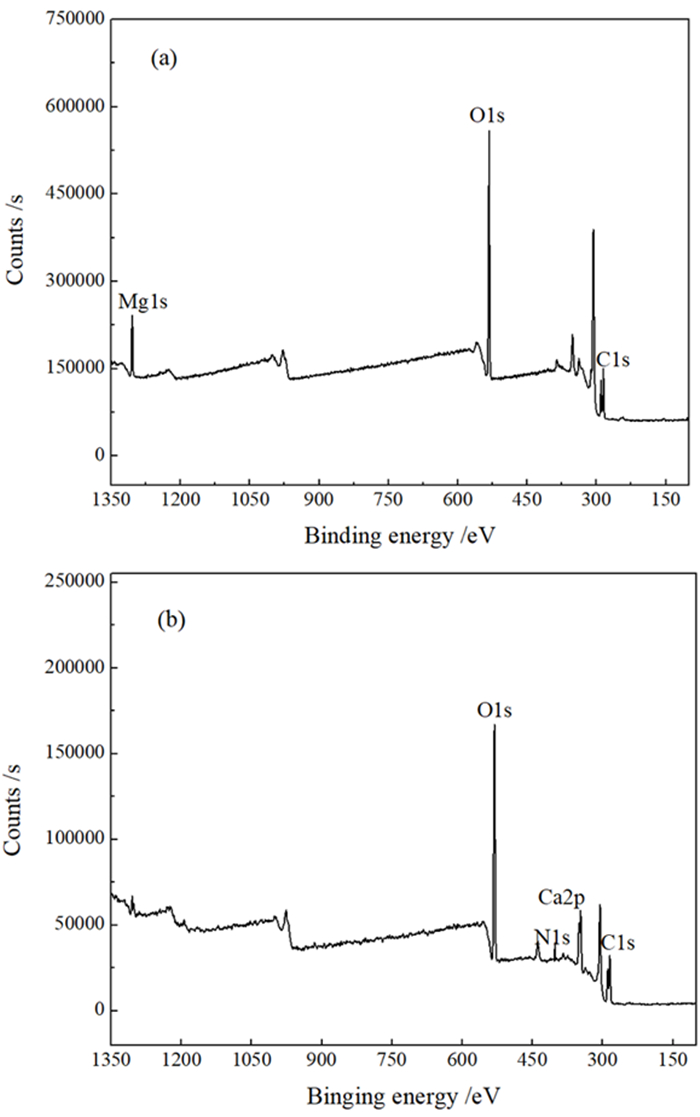
矿物 检测条件 元素含量/%(结合能/eV) C 1s O 1s Mg 1s Ca 2p N 1s 菱镁矿 自然条件 44.40 (284.80) 48.52 (531.38) 7.08(1304.43) — — pH 11.0、BAPTA 80 mg/L 45.27 (284.80) 47.61 (531.64) 7.01(1304.40) — 0.11(402.02) 偏差 0.87(0.00) -0.91(0.26) -0.07(-0.03) 0.11(402.02) 方解石 自然条件 41.92 (284.80) 50.76 (531.10) — 9.32(347.46) — pH 11.0、BAPTA 80 mg/L 45.80 (284.80) 44.31 (531.27) — 6.18(347.93) 3.71(402.06) 偏差 3.88(0.00) -6.45(0.17) — -3.15(0.47) 3.71(402.06) 标注: “―”表示检测过程中元素含量低于0.10%的分析分辨率,无法通过试验检测发现。 -
[1] HU Y, CHEN P, SUN W. Study on quantitative structure-activity relationship of quaternary ammonium salt collectors for bauxite reverse flotation [J]. Minerals Engineering, 2012, 26: 24-33. doi: 10.1016/j.mineng.2011.10.007
[2] 姚金. 含镁矿物浮选体系中矿物的交互影响研究[D]. 沈阳: 东北大学, 2014.
YAO J. Research on the reciprocal influences among magnesium-containing ores in flotation[D]. Shenyang: Northeastern University, 2014.
[3] 李虎龙. 研究菱镁矿矿床地质特征及其应用[J]. 世界有色金属, 2019(5): 284-286. doi: 10.3969/j.issn.1002-5065.2019.05.175
LI H L. Study on geological characteristics of magnesite deposit and its application[J]. World Nonferrous Metals, 2019(5): 284-286. doi: 10.3969/j.issn.1002-5065.2019.05.175
[4] 邓志杰. 世界镁的生产及发展动态[J]. 中国金属通报, 2000(21): 9-10. https://www.cnki.com.cn/Article/CJFDTOTAL-JSTB200021004.htm
DENG Z J. Production and development of magnesium in the world[J]. China Metal Bulletin, 2019(21): 9-10. https://www.cnki.com.cn/Article/CJFDTOTAL-JSTB200021004.htm
[5] YUN Z, GUO Z. A technology of preparing honeycomb-like structure MgO from low grade magnesite [J]. International Journal of Mineral Processing, 2014, 126: 35-40. doi: 10.1016/j.minpro.2013.11.006
[6] KOZHEVNIKOV E, KROPANEV S, BARAANOVSKII N. Beneficiation of dolomites [J]. Raw Material, 1973(3): 19-21.
[7] HAN J, FU D, CUO J, et al. Volatilization and condensation behavior of magnesium vapor during magnesium production via a silicothermic process with magnesite [J]. Vacuum, 2021, 189: 110227. doi: 10.1016/j.vacuum.2021.110227
[8] ZHANG Z, MAO J. Effect of sodium oleate on flotation of magnesite[J]. Journal of Wuhan University of Technology-Materials Science, 1992(4): 60-70.
[9] GENCE N. Wetting behavior of magnesite and dolomite surfaces[J]. Applied Surface Science, 2006, 252: 3744-3750. doi: 10.1016/j.apsusc.2005.05.053
[10] GENCE N, OZBAY N. pH dependence of electrokinetic behavior of dolomite and magnesite in aqueous electrolyte solutions[J]. Applied Surface Science, 2006, 252: 8057-8061. doi: 10.1016/j.apsusc.2005.10.015
[11] TANG Y, YIN W, KELEBEK S. Selective flotation of magnesite from calcite using potassium cetyl phosphate as a collector in the presence of sodium silicate[J]. Minerals Engineering, 2020, 146: 106154. doi: 10.1016/j.mineng.2019.106154
[12] 王金良, 杨秀花. 菱镁矿除钙可选性研究[J]. 中国非金属矿工业导刊, 2010(04): 28-31. https://www.cnki.com.cn/Article/CJFDTOTAL-LGFK201004011.htm
WANG J L, YANG X H. Study on separability of dolomite from magnesite ores[J]. China Non-metallic Mining Industry Herald, 2010(4): 28-31. https://www.cnki.com.cn/Article/CJFDTOTAL-LGFK201004011.htm
[13] YANG B, ZHU Z, SUN H, et al. Improving flotation separation of apatite from dolomite using PAMS as a novel eco-friendly depressant[J]. Minerals Engineering, 2020, 156: 106492. doi: 10.1016/j.mineng.2020.106492
[14] ZHU Z, WANG D, YANG B, et al. Effect of nano-sized roughness on the flotation of magnesite particles and particle-bubble interactions[J]. Minerals Engineering, 2020, 151: 106340. doi: 10.1016/j.mineng.2020.106340
[15] JOHN B, EMMA R. Calcium in biological systems[J]. Advances in Inorganic Chemistry, 2009, 61: 251-366.
[16] KOIDE Y, YAMADA K. Selective flotation of metal ions by using chelating surfactants[J]. Journal of Japan Oil Chemists' Society, 1990, 39: 736-743. doi: 10.5650/jos1956.39.10_736
[17] LIU C, ZHANG W, SONG S, et al. Flotation separation of smithsonite from calcite using 2-phosphonobutane-1, 2, 4-tricarboxylic acid as a depressant[J]. Powder Technology, 2019, 352: 11-15. doi: 10.1016/j.powtec.2019.04.036
[18] YIN W, YANG B, FU Y, et al. Effect of calcium hypochlorite on flotation separation of covellite and pyrite[J]. Powder Technology, 2019, 343: 578-585. doi: 10.1016/j.powtec.2018.11.048
[19] SANTANA A, PERES A. Reverse magnesite flotation[J]. Minerals Engineering 2001, 14: 107-111. doi: 10.1016/S0892-6875(00)00164-3
[20] SUN R, LIU D, LI Y, et al. Influence of lead ion pretreatment surface modification on reverse flotation separation of fluorite and calcite[J]. Minerals Engineering, 2021, 171: 107077. doi: 10.1016/j.mineng.2021.107077
[21] ANFRUNS J, KITCHEENER J. Rate of capture of small particles in flotation[J]. Transactions of the Institution of Mining and Metallurgy C: Mineral Processing and Extractive Metallurgy, 1977, 86: 9-15.
[22] ZHOU Z, XU Z, FINCH J, et al. Role of hydrodynamic cavitation in fine particle flotation[J]. International Journal of Mineral Processing, 1997, 51: 139-149. doi: 10.1016/S0301-7516(97)00026-4
[23] BAER D, MARMORSTEIN A, WILLIFORD R, et al. Comparison Spectra for Calcite by XPS[J]. Surface Science Spectra, 1992(1): 80-86.
[24] LIN B, WANG R, LIN J, et al. Effect of chlorine on the chemisorptive properties and ammonia synthesis activity of alumina-supported catalysts[J]. Catalysis Letters, 2011, 141: 1557-1568. doi: 10.1007/s10562-011-0658-3
[25] SIMON M, HARRISON D, BERS M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 1987, 925(2): 133-143. doi: 10.1016/0304-4165(87)90102-4
[26] SUN H, YIN W, YAO J. Study of selective enhancement of surface hydrophobicity on magnesite and quartz by N, N-Dimethyloctadecylamine: Separation test, adsorption mechanism, and adsorption model[J]. Applied Surface Science, 2022, 152482.
-



 下载:
下载: