Preparation and Growth Mechanism of Nesquehonite Crystal Crystals by Bubble Template Method with Magnesite
-
摘要:
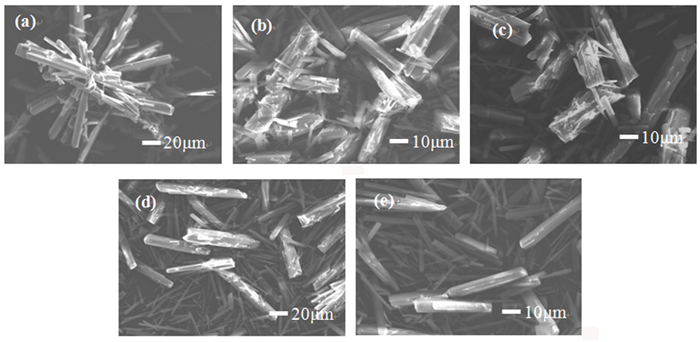
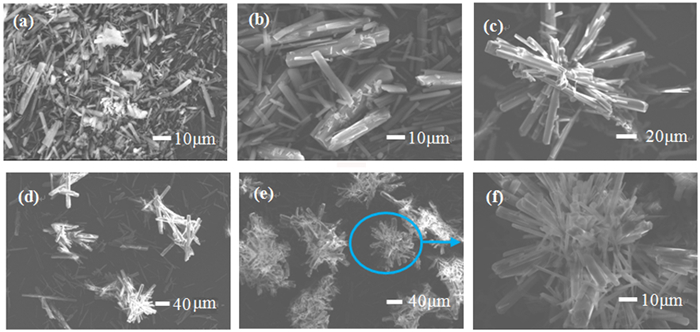
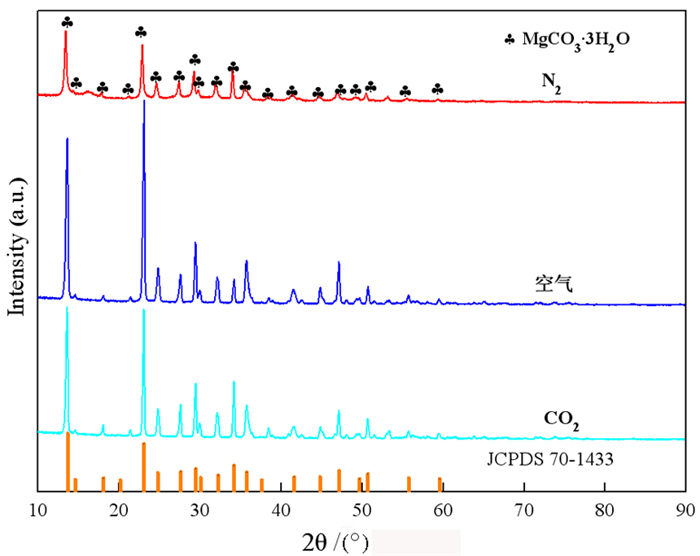
以菱镁矿为原料,采用气泡模板法制备三水碳酸镁(MgCO3·3H2O)晶体,探究CO2、空气、N2三种气泡对晶体物相组成与形貌的影响。结果表明: CO2、空气、N2辅助下,所得产物为形貌不同的三水碳酸镁晶体,N2气泡对产物形貌具有调控作用。当通入尺寸为1 μm的N2气泡60 min时,获得光滑棒状与放射状晶体; N2气泡通入时间为120 min时,产物为多面体状与放射状晶体。N2气泡充当泡界模板,晶体沿着气泡表面生长形成新的棒状三水碳酸镁分枝,最终构筑成不规则多面体状和放射状三水碳酸镁晶体。
Abstract:Nesquehonite (MgCO3·3H2O) crystals were prepared by the method for bubble template using magnesite as raw material. The effects of CO2, air and N2 bubbles on the phase composition and morphology of the crystals were investigated. The results showed that the products obtained are nesquehonite crystals with different morphologies under the assistance of CO2, air and N2. When N2 bubbles with a size of 1 μm are introduced for 60 min, the obtained products are smooth rod-like and radial crystals; when bubbles are introduced for 120 min, the obtained products are polyhedral and radial crystals. The N2 bubbles were used as bubble boundary templates, and the crystals grew along the surface of the bubbles to form new rod-like branches of nesquehonite. Finally, irregular polyhedral and radial nesquehonite crystals were constructed.
-
Key words:
- magnesite /
- nesquehonite /
- bubble /
- mechanism
-

-
表 1 菱镁矿与其煅烧所得轻烧氧化镁的化学组成
Table 1. Chemical composition of the magnesite and light-burned magnesia
/% 组分 MgO SiO2 CaO TFe 宽甸菱镁矿 47.61 0.66 0.50 - 轻烧氧化镁 82.70 7.86 1.50 0.34 -
[1] BEINLICH A, AUSTRHEIM H. In situ sequestration of atmospheric CO2 at low temperature and surface cracking of serpentinized peridotite in mine shafts[J]. Chemical Geology, 2012, 332/333: 32-44. doi: 10.1016/j.chemgeo.2012.09.015
[2] PRONOST J, BEAUDOIN G, LEMIEUX J M, et al. CO2-depleted warm air venting from chrysotile milling waste (Thetford Mines, Canada): Evidence for in-situ carbon capture from the atmosphere[J]. Geology, 2012, 40: 275-278.
[3] 马瑾, LAMY-CHAPPUIS BENOIT, 胥蕊娜, 等. 地质封存过程中饱和二氧化碳水溶液在岩心中矿化反应研究[J]. 工程热物理学报, 2015, 36(3): 627-630. https://www.cnki.com.cn/Article/CJFDTOTAL-GCRB201503037.htm
MA J, LAMY-CHAPPUIS BENOIT, XU R N, et al. Alteration of calcareous sandstone due to chemical reaction with injected carbon dioxide[J]. Journal of Engineering Thermophysics, 2015, 36(3): 627-630. https://www.cnki.com.cn/Article/CJFDTOTAL-GCRB201503037.htm
[4] 莫淳, 廖文杰, 梁斌, 等. 工业固废活化钾长石-CO2矿化提钾的生命周期碳排放与成本评价[J]. 化工学报, 2017, 68(6): 2051-2059. https://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201706033.htm
MO C, LIAO W J, LIANG B, et al. Life-cycle greenhouse gas emissions and cost of potassium extraction and CO2 mineralization via K-feldspar—industrial solid waste calcination[J]. CIESC Journal, 2017, 68(6): 2051-2059. https://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201706033.htm
[5] YAO J, SUN H, HAN F, et al. Enhancing selectivity of modifier on magnesite and dolomite surfaces by pH control[J]. Powder Technology, 2020, 362: 698-706. doi: 10.1016/j.powtec.2019.12.040
[6] YAO J, SUN H, YANG B, et al. Selective co-adsorption of a novel mixed collector onto magnesite surface to improve the flotation separation of magnesite from dolomite[J]. Powder Technology, 2020, 371: 180-189. doi: 10.1016/j.powtec.2020.05.098
[7] 王晗, 朱辰, 张猛龙, 等. 富镁尾矿封存二氧化碳的试验地球化学研究[J]. 高校地质学报, 2020, 26(3): 303-312. https://www.cnki.com.cn/Article/CJFDTOTAL-GXDX202003007.htm
WANG H, ZHU C, ZHANG M L, et al. CO2 absorption and precipitation study using magnesium-based tailings from salt lake potassium production[J]. Geological Journal of China Universities, 2020, 26(3): 303-312. https://www.cnki.com.cn/Article/CJFDTOTAL-GXDX202003007.htm
[8] ZHANG Z, ZHENG Y, ZHANG J, et al. Synthesis and shape evolution of monodisperse basic magnesium carbonate microspheres[J]. Crystal Growth & Design, 2007, 7: 337-342.
[9] ZHANG Z, ZHENG Y, ZHANG J, et al. Magnesium oxide microspheres as a packing material for the separation of basic compounds in normal-phase liquid chromatography[J]. Journal of Chromatography A, 2007, 1165: 116-121. doi: 10.1016/j.chroma.2007.07.072
[10] WANG Y L, LIU J Y, SHI T J, et al. Synthesis and pore structure construction mechanism of porous nesquehonite[J]. Powder Technology, 2022, 398: 117154. doi: 10.1016/j.powtec.2022.117154
[11] SKLIROS V, TSAKIRIDIS P, PERRAKI M. A combined Raman, Fourier transform infrared, and X-ray diffraction study of thermally treated nesquehonite[J]. Journal of Raman Spectroscopy, 2019, 51(9): 1445-1453.
[12] HAMILTON L, WILSON A, MORGAN B, et al. Accelerating mineral carbonation in ultramafic mine tailings via direct CO2 reaction and heap leaching with potential for base metal enrichment and recovery[J]. Economic Geology, 2020, 115(2): 303-323. doi: 10.5382/econgeo.4710
[13] CAO X L, REN Q Y, YANG Y K, et al. A new environmentally-friendly route to in situ form a high-corrosion-resistant nesquehonite film on pure magnesium[J]. RSC Advances, 2020, 10: 35480-35489. doi: 10.1039/D0RA04423G
[14] CHENG W, ZHANG C, CHENG H, et al. Effect of ethanol on the crystallization and phase transformation of MgCO3·3H2O in a MgCl2-CO2-NH3·H2O system[J]. Powder Technology, 2018, 335: 164-170. doi: 10.1016/j.powtec.2018.04.063
[15] YANG C, SONG X, SUN S, et al. Effects of sodium dodecyl sulfate on the oriented growth of nesquehonite whiskers[J]. Advanced Powder Technology, 2013, 24(3): 585-592. doi: 10.1016/j.apt.2012.10.005
[16] 高玉娟, 徐梦旭, 田贵山, 等. 反应物浓度对三水碳酸镁晶体的形貌调控[J]. 山东理工大学学报(自然科学版), 2021, 35(2): 28-30.
GAO Y J, XU M X, TIAN G S, et al. Reactant concentration on the morphology control of nesquehonite crystal[J]. Journal of Shandong University of Technology(Natural Science Edition), 2021, 35(2): 28-30.
[17] 闫平科, 梁帅, 高玉娟, 等. 高长径比三水碳酸镁晶须的合成研究[J]. 人工晶体学报, 2012, 41(5): 1345-1351. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201201037.htm
YAN P K, LIANG S, GAO Y J, et al. Research on the synthesis of nesquehonite whisker with high aspect ratios[J]. Journal of Synthetic Crystals, 2012, 41(5): 1345-1351. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201201037.htm
[18] 闫平科, 薛国梁, 高玉娟, 等. 表面活性剂对三水碳酸镁晶须形貌的影响研究[J]. 硅酸盐通报, 2013, 32(9): 1729-1732+1740. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201309007.htm
YAN P K, XUE G L, GAO Y J, et al. Study on the effects of different surface-active agent on the morphology of nesquehonite whisker[J]. Bulletin of the Chinese Ceramic Society, 2013, 32(9): 1729-1732+1740. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201309007.htm
[19] 吴丹, 王玉琪, 武海虹, 等. 三水碳酸镁合成与形貌演变过程研究[J]. 人工晶体学报, 2014, 43(3): 606-613. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201403027.htm
WU D, WANG Y Q, WU H H, et al. Research on preparation and morphology evolution of magnesium carbonate tri-hydrate[J]. Journal of Synthetic Crystals, 2014, 43(3): 606-613. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201403027.htm
[20] 陈娟, 黄志良, 陈常连, 等. 反应温度和反应时间对三水碳酸镁晶须长径比的影响[J]. 武汉工程大学学报, 2017, 39(1): 54-58. https://www.cnki.com.cn/Article/CJFDTOTAL-WHHG201701009.htm
CHEN J, HUANG Z L, CHEN C L, et al. Influence of temperature and time on aspect ratios of magnesium carbonate trihydrate whiskers[J]. Journal of Wuhan Institute of Technology, 2017, 39(1): 54-58. https://www.cnki.com.cn/Article/CJFDTOTAL-WHHG201701009.htm
[21] WANG Y L, LIU J Y, SHI T J, et al. Preparation, properties and phase transition of mesoporous hydromagnesite with various morphologies from natural magnesite[J]. Powder Technology, 2020, 364: 822-830. doi: 10.1016/j.powtec.2020.01.090
[22] 王余莲, 印万忠, 张夏翔, 等. 大长径比三水碳酸镁晶须的制备及晶体生长机理[J]. 硅酸盐学报, 2018, 46(7): 938-945. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201807009.htm
WANG Y L, YIN W Z, ZHANG X X, et al. Preparation and growth mechanism of nesquehonite whiskers with large aspect ratio[J]. Journal of the Chinese Ceramic Society, 2018, 46(7): 938-945. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201807009.htm
[23] 王余莲, 印万忠, 李昂, 等. 氯化钙对三水碳酸镁晶体结晶过程的影响[J]. 矿产保护与利用, 2018(5): 110-114. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=96a74a63-1f44-45e5-a3e7-56d29b268c44
WANG Y L, YIN W Z, LI A, et al. Influence of calcium chloride on crystallization process of nesquehonite crystals[J]. Conservation and Utilization of Mineral Resources, 2018(5): 110-114. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=96a74a63-1f44-45e5-a3e7-56d29b268c44
[24] 王余莲, 印万忠, 李昂, 等. 热分解法制备三水碳酸镁晶须及其结晶动力学研究[J]. 矿产保护与利用, 2018(6): 107-113. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=39983ae4-7ecc-4e63-884c-a113c51b0c15
WANG Y L, YIN W Z, LI A, et al. Preparation and crystallization kinetics of nesquehonite whiskers with thermal decomposition method[J]. Conservation and Utilization of Mineral Resources, 2018(6): 107-113. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=39983ae4-7ecc-4e63-884c-a113c51b0c15
[25] DING W, OUYANG J, YANG H. Synthesis and characterization of nesquehonite (MgCO3·3H2O) powders from natural talc[J]. Powder Technology, 2016, 292: 169-175. doi: 10.1016/j.powtec.2016.01.037
[26] HARRISON A L, MAVROMATIS V, OELKERS E H, et al. Solubility of the hydrated Mg-carbonates nesquehonite and dypingite from 5 to 35 ℃: Implications for CO2 storage and the relative stability of Mg-carbonates[J]. Chemical Geology, 2019, 504: 123-135. doi: 10.1016/j.chemgeo.2018.11.003
[27] 陈敏, 李月圆, 王健东, 等. 利用菱镁矿制备碳酸镁晶须[J]. 硅酸盐学报, 2009, 37(10): 1649-1653. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB200910013.htm
CHEN M, LI Y Y, WANG J D, et al. Preparation of magnesium carbonate whiskers by magnesite[J]. Journal of the Chinese Ceramic Society, 2009, 37(10): 1649-1653. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB200910013.htm
[28] 欧龙, 刘家祥. 重镁水中通入空气制备三水碳酸镁晶须[J]. 硅酸盐学报, 2016, 44(1): 104-111. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201601017.htm
OU L, LIU J X. Preparation of Nesquehonite Whiskers Through Bubbling Air into Magnesium Bicarbonate Solution[J]. Journal of the Chinese Ceramic Society, 2016, 44(1): 104-111. https://www.cnki.com.cn/Article/CJFDTOTAL-GXYB201601017.htm
[29] 王杰, 汪莉, 贺拴玲, 等. Mg和Zr共掺杂TiO2对光催化还原CO2的影响[J]. 应用化工, 2019, 48(12): 2821-2826. https://www.cnki.com.cn/Article/CJFDTOTAL-SXHG201912005.htm
WANG J, WAGN L, HE S L, et al. Effect of photocatalytic reduction of carbon dioxide by Mg-Zr Co-doped nano TiO2[J]. Applied Chemical Industry, 2019, 48(12): 2821-2826. https://www.cnki.com.cn/Article/CJFDTOTAL-SXHG201912005.htm
[30] 李停停, 李国栋, 钟祥熙, 等. Au掺杂浓度对WO3微米球NO2气敏特性的影响[J]. 中国有色金属学报, 2019(1): 81-90. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201901010.htm
LI T T, LI G D, ZHONG X X, et al. Effect of Au doping concentration on NO2 gas sensing properties of WO3 microspheres[J]. The Chinese Journal of Nonferrous Metals, 2019(1): 81-90. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201901010.htm
[31] 钟祥熙, 沈岩柏, 李停停, 等. 基于硅藻土多孔陶瓷衬底制备SnO2纳米线及其低温H2S气敏性能[J]. 中国有色金属学报, 2020, 30(10): 2350-2359. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ202010011.htm
ZHONG X X, SHEN Y B, LI T T, et al. Low-temperature H2S sensing properties of SnO2 nanowiresgrown on diatomite-based porous ceramic substrate[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(10): 2350-2359. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ202010011.htm
[32] 王玉江, 黄威, 黄玉炜, 等. SiC/Fe3O4/rGO复合材料的制备及吸波性能[J]. 材料导报, 2019, 33(10): 1624-1629. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201910008.htm
WANG Y J, HUANG W, HUANG Y W, et al. Preparation and Microwave Absorbing Properties of SiC /Fe3O4 /rGO Composite Materials[J]. Materials Reports, 2019, 33(10): 1624-1629. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201910008.htm
[33] 薛国梁. 三水碳酸镁晶体形貌调控研究[D]. 阜新: 辽宁工程技术大学, 2015.
XUE G L, Study on morphological control of nesquehonite crystal[D]. Fuxin: Liaoning Technical University, 2015.
[34] 高玉娟, 闫平科, 王宇林, 等. 放射状三水碳酸镁晶体合成及生长机理研究[J]. 人工晶体学报, 2014, 43(4): 886-892. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201404037.htm
GAO Y J, YAN P K, WANG Y L, et al. Study on the synthesis and growth mechanism of the radial nesquehonite cystal[J]. Journal of Synthetic Crystals, 2014, 43(4): 886-892. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201404037.htm
[35] 闫小星, 李云飞, 薛冬峰, 等. 基于水合碳酸镁结晶过程的化学键分析[J]. 人工晶体学报, 2007, 36(5): 991-999. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT200705009.htm
YAN X X, LI Y F, XUE D F, et al. Bonding analysis on the crystallization of magnesium carbonate hydrates[J]. Journal of Synthetic Crystals, 2007, 36(5): 991-999. https://www.cnki.com.cn/Article/CJFDTOTAL-RGJT200705009.htm
[36] YANG Y C, YU H H, WANG X Y. Facile synthesis of basic magnesium carbonate with different morphology[J]. Advanced Materials Research, 2012, 554/555/556: 575-579.
[37] 马隆飞. 复配捕收剂在低阶煤/水界面的黏附铺展行为及机理研究[D]. 徐州: 中国矿业大学, 2020.
MA L F. Study on the attachment and spreading behavior and mechanism of compound collector at the low rank coal/water interface[D]. Xuzhou: China University of Mining and Technology, 2020.
[38] 陈娟. 碱式碳酸镁晶形控制及其生长机理研究[D]. 武汉: 武汉工程大学, 2017.
CHEN J. Research on crystal morphology control and growth mechanism of basic magnesium carbonate[D]. Wuhan: Wuhan Institute of Technology, 2017.
[39] CHENG X, HE Q, LI J, et al. Control of pore size of the bubble-template porous carbonated hydroxyapatite microsphere by adjustable pressure[J]. Crystal Growth & Design, 2009, 9(6): 2770-2775.
[40] CHEN B D, CILLIERS J J, DAVEY R J, et al. Templated nucleation in a dynamic environment: Crystallization in foam lamellae[J]. Journal of the American Chemical Society, 1998, 120(7): 1625-1626. doi: 10.1021/ja973069o
-



 下载:
下载: