Preparation of High-purity CaCO3 from Phosphogypsum by CO2 Mineralization and Product Control
-
摘要:
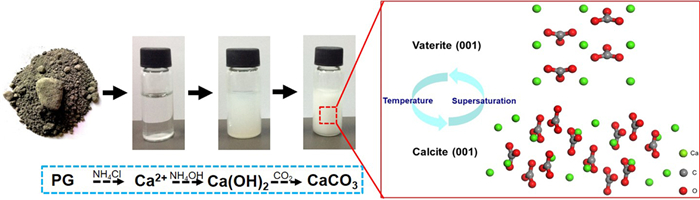
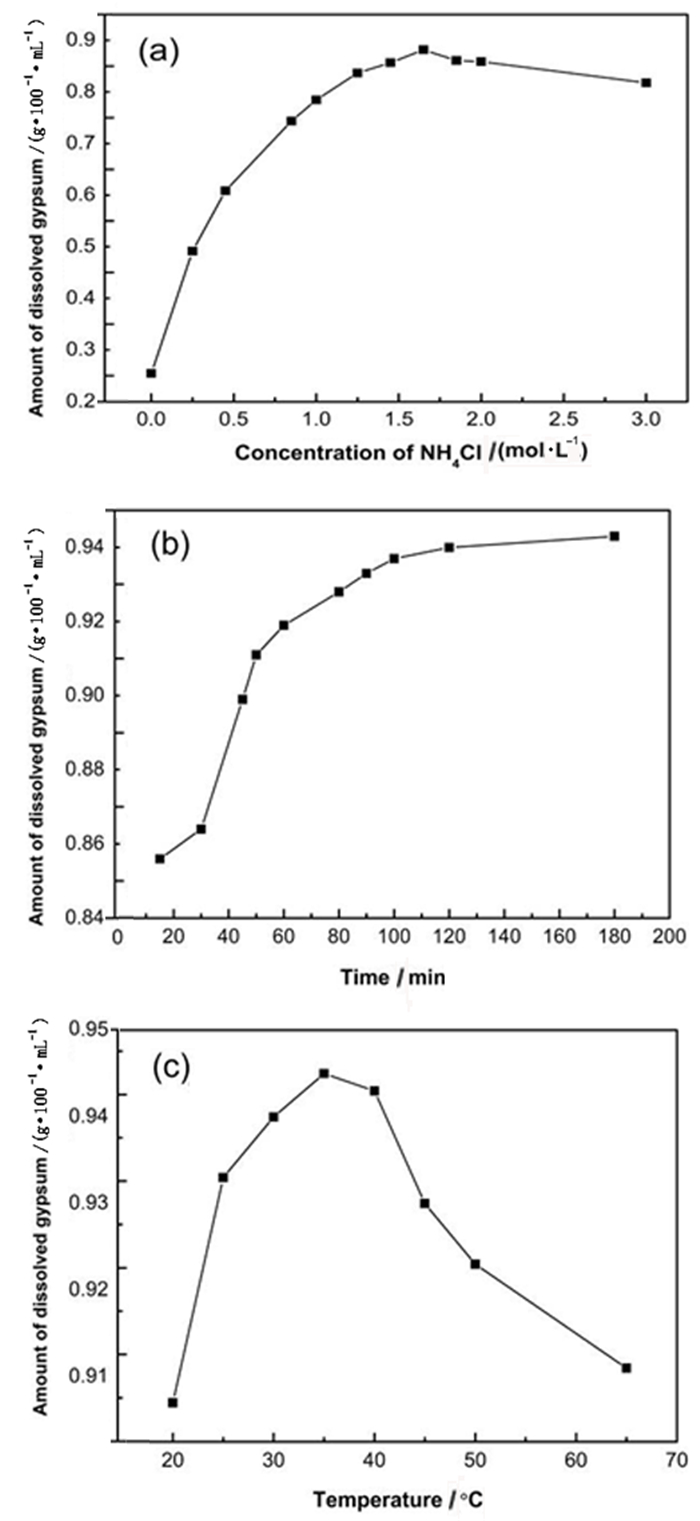
针对CO2减排与磷石膏资源化利用的巨大压力,基于CO2矿化与磷石膏利用过程耦合的学术思想,以磷石膏为原料,研究氯化铵体系中氨水强化磷石膏浸出液制备高纯CaCO3的反应过程。试验过程中系统讨论了不同工艺条件对磷石膏中二水硫酸钙浸出的影响;并就钙离子浸出液在不同条件下所得碳酸化产物的晶型与形貌进行了系统表征。结果表明:在优化的工艺条件下磷石膏中二水硫酸钙的浸出量为0.945×10-2 g/mL;所得碳酸化产物为球形球霰石,纯度99.80%,白度99.2%,碳酸化产物的基本性能满足普通工业沉淀碳酸钙标准(HG/T 2226—2010)中的指标要求;在碳酸化过程中通过工艺条件的调控成功实现球霰石和方解石与球霰石混合晶相的可控制备;通过化学分析揭示了磷石膏制备高纯碳酸钙的反应过程。该研究为磷石膏的资源化利用与高纯碳酸钙的制备提供了新的思路。
Abstract:To meet the demands on low CO2 emission and resource utilization of phosphogypsum, this paper is guided by the academic principle of coupling CO2 mineralization and phosphogypsum utilization, exploring the reaction of ammonia-strengthened phosphogypsum leaching solution to produce high-purity CaCO3 in a NH4Cl-H2O system. The effects of process parameters on the leaching efficiency of CaSO4·2H2O in phosphogypsum were discussed systematically. The crystal phase and morphology of the carbonated product were characterized. The maximum value of dissolved CaSO4·2H2O was 0.945 g/100 mL with the mild reaction conditions. Pure spherical vaterite was produced. The content of calcium carbonate in the product was 99.80% and the whiteness of the product was 99.2%, which satisfied HG/T 2226-2010. The product mixed with cubic calcite and spherical vaterite could be formed by adjusting the reaction conditions. A probable reaction process of CaCO3 production and polymorphism transformation was also proposed through chemical analysis of the liquid-solid-gas reaction. This paper provides a new idea for resource utilization of phosphogypsum and preparation of high purity calcium carbonate.
-
Key words:
- phosphogypsum /
- mineralization /
- CaCO3 /
- NH4Cl /
- product control
-

-
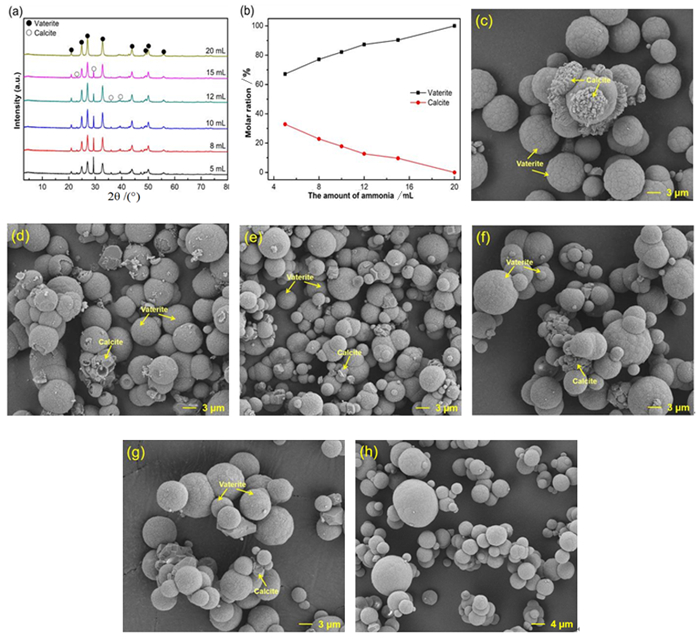
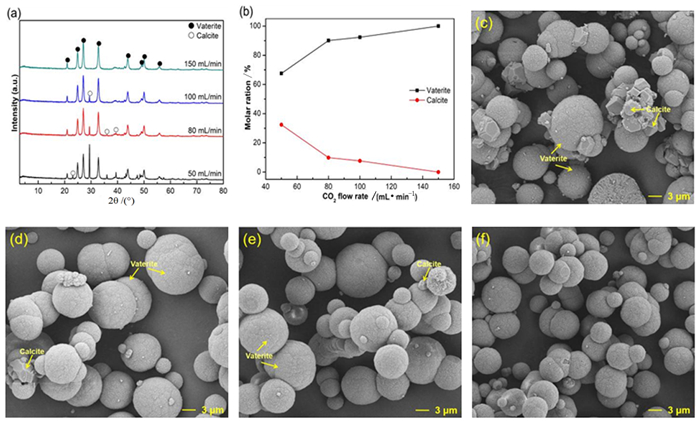
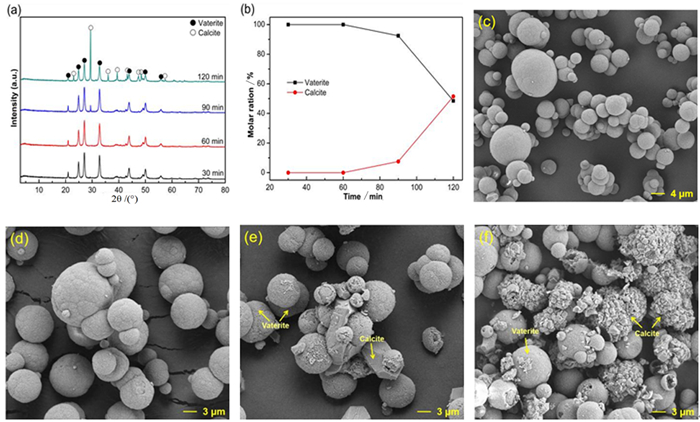
表 1 钙离子浸出液碳化的影响因素及取值
Table 1. Factors and levels of carbonation experiments for Ca2+ leaching solution
序号 氨水的量/mL CO2流速/(mL·min-1) 温度/℃ 时间/min 1 5 150 30 60 2 8 150 30 60 3 10 150 30 60 4 12 150 30 60 5 15 150 30 60 6 20 150 30 60 7 20 50 30 60 8 20 80 30 60 9 20 100 30 60 10 20 150 20 60 11 20 150 30 60 12 20 150 40 60 13 20 150 50 60 14 20 150 60 60 15 20 150 80 60 16 20 150 100 60 17 20 150 30 30 18 20 150 30 60 19 20 150 30 90 20 20 150 30 120 -
[1] WU F H, REN Y H, QU G F, et al. Utilization path of bulk industrial solid waste: a review on the multi-directional resource utilization path of phosphogypsum[J]. Journal of Environmental Management, 2022, 313: 114957. doi: 10.1016/j.jenvman.2022.114957
[2] TAYIBI H N, CHOURA MH, FELIX A, et al. Environmental impact and management of phosphogypsum[J]. Journal of Environmental Management, 2009, 90: 2377-2386. https://www.sciencedirect.com/science/article/pii/S0301479709000784
[3] DU M X, WANG JM, DONG FQ, et al. The study on the effect of flotation purification on the performance of alpha-hemihydrate gypsum prepared from phosphogypsum[J]. Scientific Reports, 2022, 12: 95. doi: 10.1038/s41598-021-04122-w
[4] MIN CD, SHI Y, LIU ZX. Properties of cemented phosphogypsum (PG) backfill in case of partially substitution of composite portland cement by ground granulated blast furnace slag[J]. Construction and Building Materials, 2021, 305: 124786. doi: 10.1016/j.conbuildmat.2021.124786
[5] MESKINI S, REMMAL T, EJJAOUANI H, et al. Formulation and optimization of a phosphogypsum-fly ash-lime composite for road construction: a statistical mixture design approach[J]. Construction and Building Materials, 2022, 315: 125786. doi: 10.1016/j.conbuildmat.2021.125786
[6] HUANG L H, LIU Y, FERREIRA J F S, et al. Long-term combined effects of tillage and rice cultivation with phosphogypsum or farmyard manure on the concentration of salts, minerals, and heavy metals of saline-sodic paddy fields in Northeast China[J]. Soil & Tillage Research, 2022, 215: 105222.
[7] BOSSOLANI J W, CRUSCIOL C A C, MORETTI L G, et al. Improving soil fertility with lime and phosphogypsum enhances soybean yield and physiological characteristics[J]. Agronomy for Sustainable Development, 2022, 42: 284.
[8] SHENG Z M, ZHOU J, SHU Z, et al. Calcium sulfate whisker reinforced non-fired ceramic tiles prepared from phosphogypsum[J]. Boletin De La Sociedad Espanola De Ceramica Y Vidrio, 2018, 57: 73-78. doi: 10.1016/j.bsecv.2017.09.005
[9] LU S Q, LAN P Q, WU S F. Preparation of nano-CaCO3 from phosphogypsum by gas-liquid-solid reaction for CO2 sorption[J]. Industrial & Engineering Chemistry Research, 2016, 55: 10172-10177. https://pubs.acs.org/doi/10.1021/acs.iecr.6b02551
[10] ARGYRIOS A, NAVARRO M, AHMAD A, et al. Valorization of phosphogypsum as a thermal energy storage material for low temperature applications[J]. Journal of Cleaner Production, 2022, 342: 130839. doi: 10.1016/j.jclepro.2022.130839
[11] JIANG G Z, WU A X, WANG Y M, et al. Determination of utilization strategies for hemihydrate phosphogypsum in cemented paste backfill: used as cementitious material or aggregate[J]. Journal of environmental management, 2022, 308: 114687-114687. doi: 10.1016/j.jenvman.2022.114687
[12] YANG J, ZENG J Y, HE X Y, et al. Sustainable clinker-free solid waste binder produced from wet-ground granulated blast-furnace slag, phosphogypsum and carbide slag[J]. Construction and Building Materials, 2022, 330: 127218. doi: 10.1016/j.conbuildmat.2022.127218
[13] SABRINA F L, MARCOS L S, SAMUEL R W, et al. Leaching of rare earth elements from phosphogypsum[J]. Chemosphere, 2022, 301: 134661-134661. doi: 10.1016/j.chemosphere.2022.134661
[14] PAN Q H, MA L P, DU W, et al. Hydrogen-enriched syngas production by lignite chemical looping gasification with composite oxygen carriers of phosphogypsum and steel slag[J]. Energy, 2022, 241: 122927. doi: 10.1016/j.energy.2021.122927
[15] ZHAO B, WANG Y Y, MA L T, et al. Adding an appropriate proportion of phosphogypsum ensured rice husk and urea composting to promote the compost as substrate utilization[J]. Bioresource Technology, 2022, 344: 126301. doi: 10.1016/j.biortech.2021.126301
[16] MAHMUT A, SONER T, BURIN K. Ultrasonic-assisted production of precipitated calcium carbonate particles from desulfurization gypsum[J]. Ultrasonics Sonochemistry, 2021, 72: 105421. doi: 10.1016/j.ultsonch.2020.105421
[17] AMINE I, BRAHIM B, BRAHIM E, et al. Phosphogypsum two-step ammonia-carbonation resulting in ammonium sulfate and calcium carbonate synthesis: effect of the molar ratio OH-/Ca2+ on the conversion process[J]. Waste and Biomass Valorization, 2022, 13: 1795-1806. doi: 10.1007/s12649-021-01600-0
[18] CHEN Q J, DING W J, SUN H J, et al. Indirect mineral carbonation of phosphogypsum for CO2 sequestration[J]. Energy, 2020, 206: 118148. doi: 10.1016/j.energy.2020.118148
[19] BOUARGANE B, BIYOUNE M G, MABROUK A, et al. Experimental Investigation of the effects of synthesis parameters on the precipitation of calcium carbonate and portlandite from moroccan phosphogypsum and pure gypsum using carbonation route[J]. Waste and Biomass Valorization, 2020, 11: 6953-6965. doi: 10.1007/s12649-019-00923-3
[20] DING WJ, CHEN Q J, SUN H J, et al. Modified phosphogypsum sequestrating CO2 and characteristics of the carbonation product[J]. Energy, 2019, 182: 224-235. doi: 10.1016/j.energy.2019.05.220
[21] YANG B J, YANG M M, WANG B N, et al. A new route to synthesize calcium carbonate microspheres from phosphogypsum[J]. Materials Research Express, 2019, 6: 045042. doi: 10.1088/2053-1591/aafadf
[22] INES H, KARIMA H N. Solubility study and valorization of phosphogypsum salt solution[J]. International Journal of Mineral Processing, 2013, 123: 87-93. doi: 10.1016/j.minpro.2013.05.008
[23] WANG D W, XU L, NAI J W, et al. Morphology-controllable synthesis of nanocarbons and their application in advanced symmetric supercapacitor in ionic liquid electrolyte[J]. Applied Surface Science, 2019, 473: 1014-1023. doi: 10.1016/j.apsusc.2018.12.256
[24] ZHANG L M, FANG X, SONG Z D, et al. Phase and morphology evolution during the solvothermal synthesis of VO2 polymorphs[J]. Inorganic Chemistry Frontiers, 2015(3): 117-124. https://pubs.rsc.org/en/content/articlelanding/2016/qi/c5qi00195a
[25] SONG K, KIM W, BANG J H, et al. Polymorphs of pure calcium carbonate prepared by the mineral carbonation of flue gas desulfurization gypsum[J]. Materials and Design, 2015, 83: 308-313. doi: 10.1016/j.matdes.2015.06.051
[26] SPANOS N, KOUTSOUKOS P G. The transformation of vaterite to calcite: effect of the conditions of the solutions in contact with the mineral phase[J]. Journal of Crystal Growth, 1998, 191: 783-790. doi: 10.1016/S0022-0248(98)00385-6
[27] BAO W J, ZHAO H T, LI H Q, et al. Process simulation of mineral carbonation of phosphogypsum with ammonia under increased CO2 pressure[J]. Journal of CO2 Utilization, 2017, 17: 125-136. doi: 10.1016/j.jcou.2016.11.012
[28] LEE M G, JANG YN, RYU K W, et al. Mineral carbonation of flue gas desulfurization gypsum for CO2 sequestration[J]. Energy, 2012, 47: 370-377. doi: 10.1016/j.energy.2012.09.009
-



 下载:
下载: