Research on the Inhibition Mechanism of Different Calcium Ion Precipitation on Molybdenum Flotation
-
摘要:
辉钼矿浮选矿浆中含有的大量钙离子,可能与
< span class="inline-formula-span" > ${\mathrm{SO}}_4^{2-} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='2024-01-0010_Z-20240401165426.png'/ > < span class="inline-formula-span" > ${\mathrm{MoO}}_4^{2-} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='2024-01-0010_Z-20240401165448.png'/ > < span class="inline-formula-span" > ${\mathrm{CO}}_3^{2-} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='2024-01-0010_Z-20240401165504.png'/ > Abstract:Molybdenum flotation pulps containing a large amount of Ca2+, which may precipitate on the surface of Molybdenum with such as
< span class="inline-formula-span" > ${\mathrm{SO}}_4^{2-} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='2024-01-0010_Z-20240402154743.png'/ > < span class="inline-formula-span" > ${\mathrm{MoO}}_4^{2-} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='2024-01-0010_Z-20240402154747.png'/ > < span class="inline-formula-span" > ${\mathrm{CO}}_3^{2-} $ < /span > < img text_id='' class='formula-img' style='display:none;' src='2024-01-0010_Z-20240402154758.png'/ > -
Key words:
- molybdenum /
- flotation /
- calcium ion precipitation /
- solution chemistry
-

-
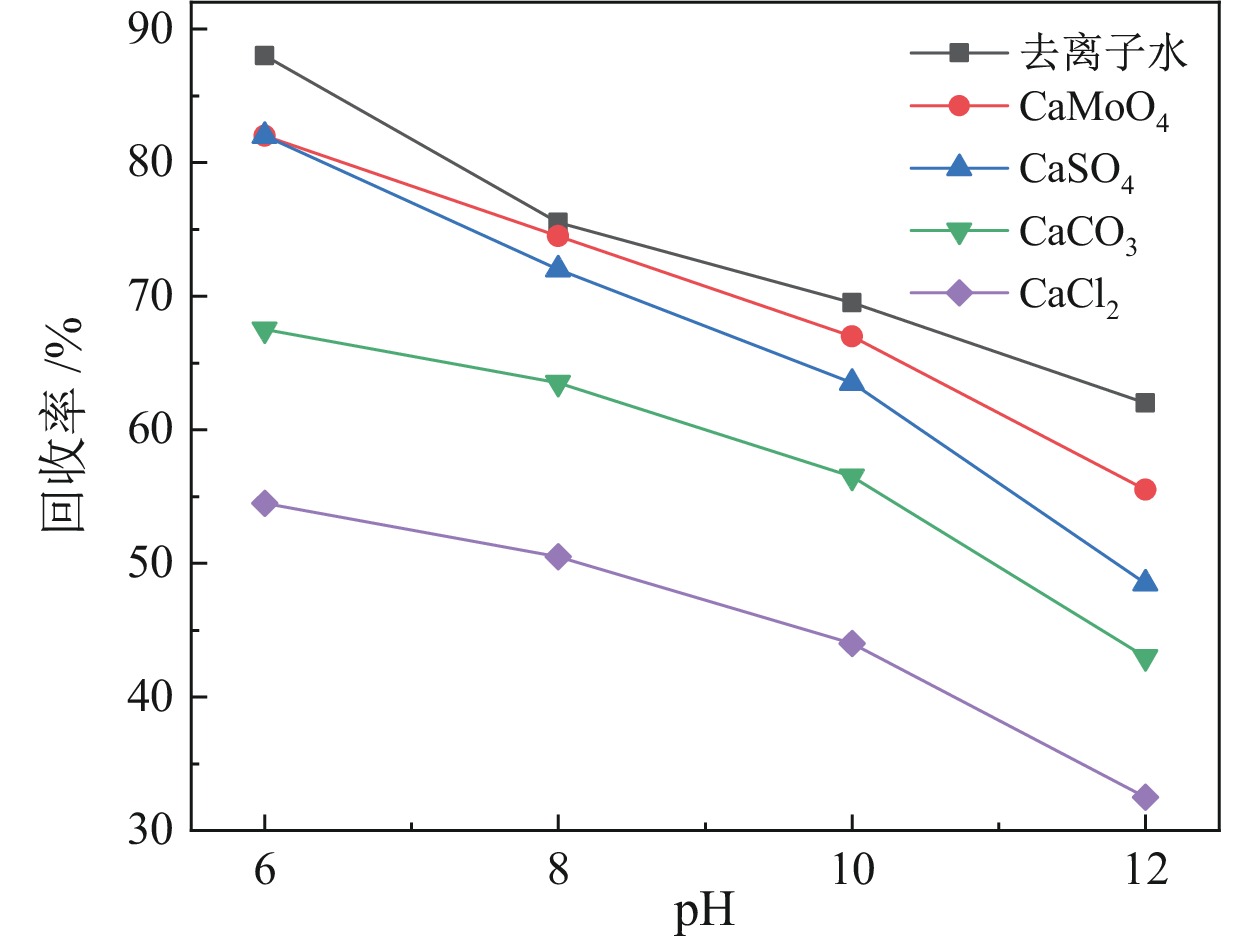
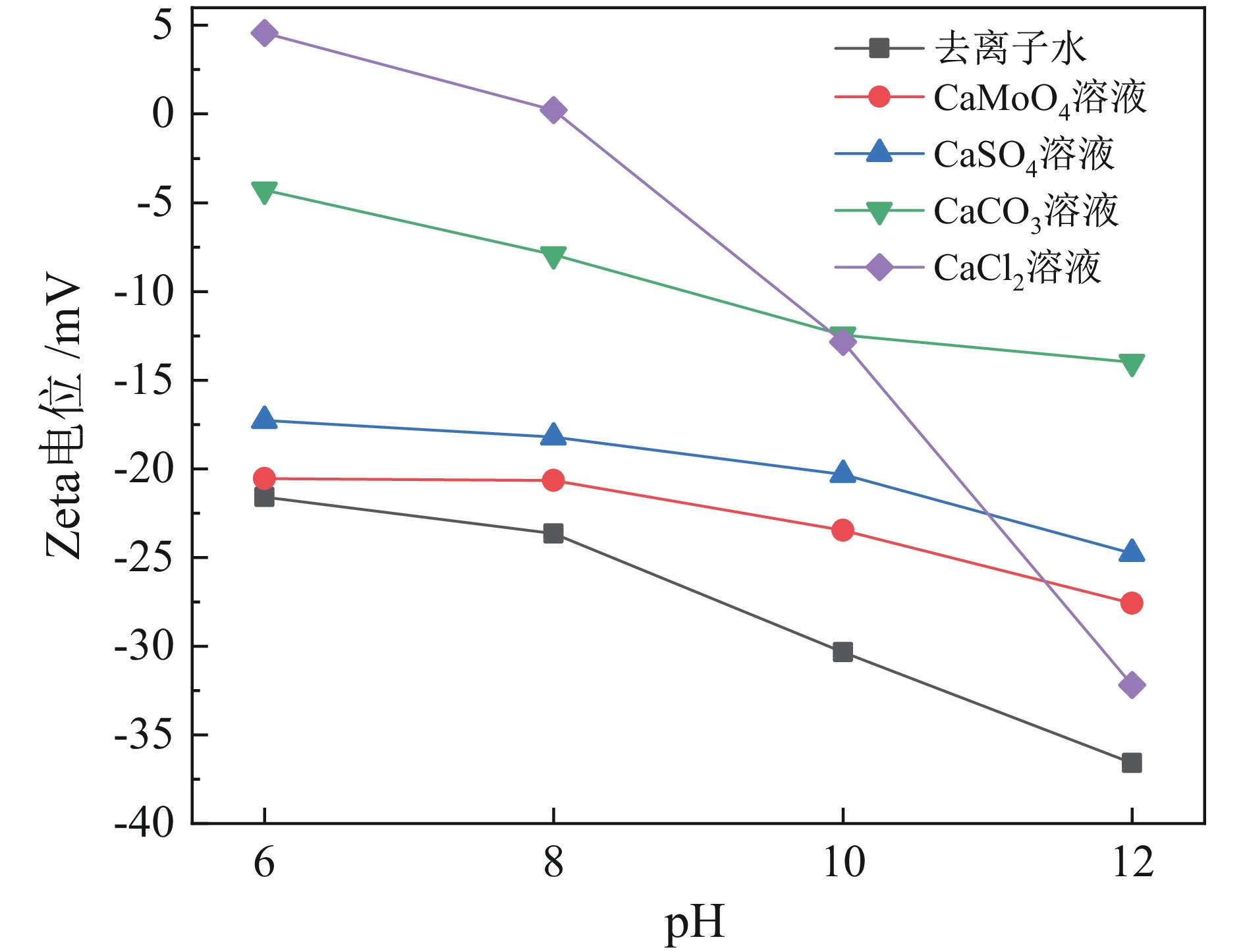
表 1 不同浮选环境的矿浆pH
Table 1. Pulp pH in different flotation environments
去离子水 CaMoO4 CaSO4 CaCO3 Ca(OH)2 6.67 7.22 7.03 9.91 11.92 -
[1] 付静波, 赵宝华. 国内外钼工业发展现状[J]. 稀有金属, 2007(1): 151−154.
FU J B, ZHAO B H. Present states of development of molybdenum industry at home and abroad[J]. Chinese Journal of Rare Metals, 2007(1): 151−154.
[2] 彭涛, 彭如清. 中国钼工业现状及发展战略[J]. 有色金属工业, 1998(10): 14−17.
PENG T, PENG R Q. The current situation and development strategy of China's molybdenum industry[J]. China Nonferrous Metals, 1998(10): 14−17.
[3] 朱欣然. 国内外钼资源供需形势分析[J]. 矿产保护与利用, 2020, 40(1): 172−178.
ZHU X R. Analysis of the supply and demand dituation of molybdenum resources at home and abroad[J]. Conservation and Utilization of Resouces, 2020, 40(1): 172−178.
[4] 孙兴家. 辉钼矿的工艺矿物性质[J]. 有色金属(选矿部分), 1982(5): 54−58, 32
SUN X J Process mineral properties of molybdenite[J]. Nonferrous Metals(Mineral Processing Section), 1982(5): 54−58, 32.
[5] 魏桢伦, 李育彪. 辉钼矿晶面各向异性及其对浮选的影响机制[J]. 矿产保护与利用, 2018(3): 31−36.
WEI Z L, LI Y B. Anisotropy of molybdenum crystal plane and its influence mechanism on flotation[J]. Conservation and Utilization of Mineral Resources, 2018(3): 31−36.
[6] 殷俊良. 国外利用海水选矿的经验[J]. 有色矿山, 1982(6): 28−32.
YIN J L. Experience of using seawater for mineral processing abroad[J]. China Mine Engineering, 1982(6): 28−32.
[7] RICRADO I J, LIZA F, LUIS A C. Effect of seawater on sulfide ore flotation: A review[J]. Mineral Processing and Extractive Metallurgy Review, 2016, 37(6): 369−384. doi: 10.1080/08827508.2016.1218871
[8] 胡静文, 王艳红, 顾帼华, 等. 选矿废水的净化处理技术及机理研究进展[J]. 矿产保护与利用, 2021, 41(4): 35−42.
HU J W, WANG Y H, GU J H, et al. Research progress on purification treatment technology and mechanism of mineral processing wastewater[J]. Conservation and Utilization of Mineral Resources, 2021, 41(4): 35−42.
[9] 李明明, 尹禹琦, 宛鹤. 选钼废水回用处理浅析[J]. 中国钼业, 2020,44(3):4-8.
LI M M, YIN Y Q, WAN H. Resue and treatment of molybdenum benefication wastewater[J]. China Molybdenum Industry, 2020, 44(3): 4−8.
[10] 阎文庆, 朱日来. 苦咸水、海水在国内外矿业中的应用[J]. 中国矿业, 2016, 25(10): 81−87+113
YAN W Q, ZHU R L. Use of salt water in domes tic and foreign mining industries[J]. China Mining Magazine, 2016, 25(10): 81−87+113.
[11] 宛鹤, 何廷树. 选钼废水性质及回用现状[J]. 中国钼业, 2016, 40(5): 11−15.
WAN H, HE T S. Properties of molybdenum benefication wastewater and its resure[J]. China Molybdenum Industry, 2016, 40(5): 11−15.
[12] HIRAJIMA T, SUYANTARA G P, ICHIKAWA O, et al. Effect of Mg2+ and Ca2+ as divalent seawater cations on the floatability of molybdenite and chalcopyrite[J]. Minerals Engineering, 2016(96): 83−93.
[13] QU J P, HE T S, BU X Z, et al. New concept on high−calcium flotation wastewater reuse[J]. Minerals, 2018(8): 496.
[14] 张作金, 陈海彬, 吴天来, 等. 我国选矿废水处理研究进展[J]. 矿产保护与利用, 2020, 40(1): 79−84.
ZHANG Z J, CHEN H B, WU T L, et al. Research progress in the treatment of mineral processing wastewater in China[J]. Conservation and Utilization of Mineral Resources, 2020, 40(1): 79−84.
[15] WAN H, YI P, SONG X W, et al. Role of improving molybdenite flotation by using aromatic hydrocarbon collector in high−calcium water: A multiscale investigation[J]. Minerals Engineering, 2023, 191: 107984.
[16] WAN H, YANG W, HE T S, et al. The influence of Ca2+ and pH on the interaction between PAHs and molybdenite edges[J]. Minerals, 2017, 7(6): 104. doi: 10.3390/min7060104
[17] QIU Z H, LIU G Y, LIU Q X, et al. Understanding the roles of high salinity in inhibiting the molybdenite flotation[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2016, 509: 123−129.
[18] LUCAY F, CISTERNAS L A, GALVEZ E D, et al. Study of the natural floatability of molybdenite fines in saline solutions and effect of gypsum precipitation[J]. Mining, Metallurgy & Exploration, 2015, 32(4): 203−208.
[19] WANG J Y, XIE L, LU Q Y, et al. Electrochemical investigation of the interactions of organic and inorganic depressants on basal and edge planes of molybdenite[J]. Journal of Colloid And Interface Science, 2020(570): 350−361.
[20] 靳强, 高鹏元, 陈宗元, 等. Visual MINTEQ软件在大学化学教学中的应用[J]. 大学化学, 2021, 36(12): 192−198.
JING Q, GAO P Y, CHEN Z Y et al. Application of Visual MINTEQ software in college chemistry teaching[J]. University Chemistry, 2021, 36(12): 192−198.
[21] PENG Y, LI Y B, LI W Q, et al. Elimination of adverse effects of seawater on molybdenite flotation using[J]. Minerals Engineering, 2020(146): 106108.
-










 下载:
下载: