Selective Separation Condition Verification and Accuracy Evaluation of Chemical Phase Analysis Method for Antimony Ore
-
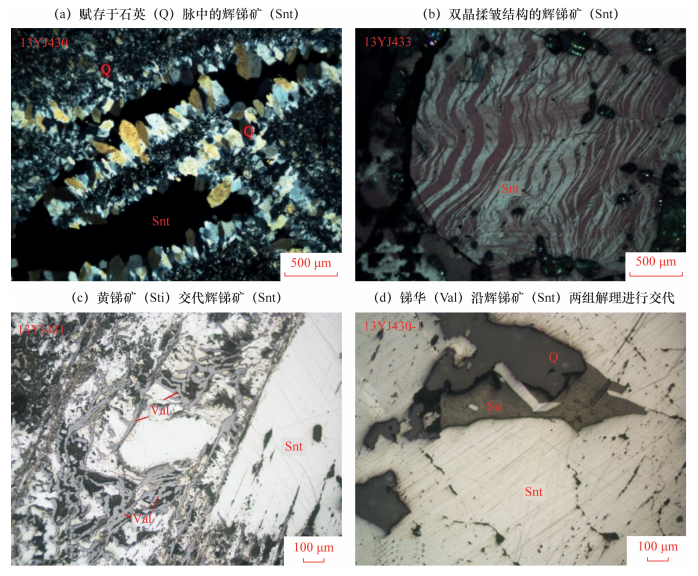
摘要: 目前我国矿石化学物相分析方法,除少数方法具有较广泛的适应性外,多数方法均是针对某一具体矿区的样品而制定,在实际应用中无法确认分析结果的准确性和选择性分离流程的适应范围。验证化学物相分析方法的选择性分离流程及方法准确度是该领域必须解决的问题。本文以我国具有代表性的锑矿类型——单锑硫化矿为研究对象,通过岩矿鉴定确定锑的主要矿物相,从试验样品中挑选验证选择分离条件所需的单矿物,在无法获得锑华单矿物的情况下,通过价态分析确定了锑华与锑酸盐混合物中锑华的比例,并通过系列单矿物选择分离对比条件试验和X射线衍射分析,确定了锑华、辉锑矿和锑酸盐等锑物相的选择性分离条件。结果表明:对于硫化锑含量大于35%的样品,锑硫化相的选择浸出时间从传统的30 min调整为40 min,硫化锑的浸出率提高了4%~6%,硫化锑相对于锑酸盐的串相率降低了45%~70%,硫化锑相浸出完全,提高了硫化锑和黄锑矿相分析的准确度。该方法适用于不同地区、不同类型(氧化矿、硫化矿)锑矿石样品的锑化学物相分析。Abstract: Currently, a few chemical phase analysis methods possess a common application, but most of them are designed for samples in a specific ore district and thus the accuracy of analytical results and the application range of a selected analytical process are not certain. Verification of chemical phase analysis by selected analytical process and method accuracy are the primary problem in this field. The representative antimony ore type, single antimony sulfide ore was chosen as the research object for the study. The main mineral phases of antimony are determined by mineral identification. When valentinite mineral cannot be acquired, the proportion of valentinite in mixing oxide ore (mixture of valentinite and antimonate) is determined by valence analysis. Moreover, the selected separation condition among valentinite, stibnite and antimonate is determined by comparison of condition experiment and X-ray Diffraction Analysis. Results show for the sample with antimony suldide content higher than 35%, the extraction time for the antimony sulfide phase should change from 30 minutes to 40 minutes. When the leaching rate of stibnite is increased by 4%-6%, the analysis error of antimonates phase can be reduced by 45%-70%. Antimony sulfide was leached completely, and the analysis accuracy of antimonite and cervantite phases improved. This method is suitable for chemical phase analysis of different types of ores (oxidized and sulfide ores) from different regions.
-

-
表 1 不同条件下各矿物相的浸出率
Table 1. The leaching rate of each mineral phase under different conditions
锑矿物相 浸出条件 混合氧化矿浸出率 (%) 辉锑矿浸出率 (%) 锑华相 2 mol/L盐酸室温振荡45 min 5.67 1.38 3 g氯化钠,2 mol/L盐酸室温振荡45 min 5.54 2.10 2 mol/L盐酸-40 g/L酒石酸室温振荡45 min 5.53 2.49 2 mol/L盐酸-40 g/L酒石酸70℃水浴振荡45 min 15.56 34.90 225 g/L酒石酸室温振荡17 h 3.11 0.84 饱和酒石酸室温振荡18 h 4.00 1.04 饱和酒石酸沸水浴振荡2 h 13.05 7.10 饱和酒石酸70℃水浴振荡2 h 5.91 3.16 锑硫化相 (辉锑矿) 25%硝酸,沸水浴30 min 1.72 95.60 1.50 92.96 9.74 93.84 8.65 64.92 0.43 95.14 2.54 85.32 1.32 89.28 1.83 95.26 黄锑矿相 (锑酸盐) 2 g硫酸钾+50 mL硝酸+15 mL硫酸消解 92.62 2.79 93.02 4.94 84.78 3.67 76.82 0.58 96.45 4.02 93.46 13.64 85.62 3.61 92.25 1.45 表 2 浸出时间对不同浓度硫化相浸出结果的影响
Table 2. Effect of leaching time on the leaching results of different concentrations of sulfide
样品编号 物相 不同浸取时间下锑的含量 (%) 30 min 40 min 50 min LT-8 浸出相 28.3 28.9 28.9 残留相 3.93 3.32 3.28 LT-9 浸出相 37.8 39.7 39.0 残留相 5.82 3.92 4.10 LT-10 浸出相 44.9 47.7 47.6 残留相 6.55 3.83 3.90 表 3 配制样品化学物相分析结果与X射线衍射分析结果的比对
Table 3. Comparison of analytical results of antimony in samples with chemical phase method and X-ray diffraction method
样品编号 锑华中Sb含量 辉锑矿中Sb含量 黄锑矿中Sb含量 化学物相4次平均值 (%) X射线衍射分析结果 (%) 化学物相4次平均值 (%) X射线衍射分析结果 (%) 相对偏差 (%) 化学物相4次平均值 (%) X射线衍射分析结果 (%) 相对偏差 (%) LT-4 0.10 - 0.85 1×0.718=0.718 16.9 0.40 - - LT-5 0.34 - 5.71 7×0.718=5.03 12.7 0.95 - - LT-6 0.61 0.30 12.01 15×0.718=10.77 10.9 1.58 1.50×0.96=1.44 9.27 LT-7 0.90 0.50 19.80 25×0.718=17.95 9.80 2.40 2.0×0.96=1.92 22.2 LT-8 1.20 1.0 29.05 41×0.718=29.44 -1.33 3.30 3.0×0.96=2.88 13.6 注:0.718为元素锑与辉锑矿的换算关系,依据该辉锑矿的电子探针分析结果w(Sb)=71.8%;0.96为元素锑与黄锑矿的换算关系,依据该黄锑矿的电子探针分析结果w(Sb)=96%。“-”表示暂未提供测量值。 表 4 试验样品配制、理论计算值和分析结果对照
Table 4. Comparison of test sample preparation, theoretical calculation and analytical values
配样序号 样品编号 锑含量 (%) 样品质量 (kg) 各矿物相中锑的理论计算值与分析结果对比 锑华w(Sb) 辉锑矿w(Sb) 黄锑矿w(Sb) 理论值 (%) 检测值 (%) 理论值 (%) 检测值 (%) 理论值 (%) 检测值 (%) LT-4 LT-0 0.87 2.95 0.12 0.10 1.05 0.85 0.41 0.40 LT-9 43.58 0.05 LT-5 LT-0 0.87 2.60 0.30 0.34 5.43 5.75 0.83 0.96 LT-9 43.58 0.40 LT-6 LT-0 0.87 2.10 0.55 0.60 11.69 12.03 1.44 1.58 LT-9 43.58 0.90 LT-7 LT-0 0.87 1.50 0.85 0.89 19.20 19.85 2.18 2.40 LT-9 43.58 1.50 LT-8 LT-0 0.87 0.80 1.20 1.26 27.97 29.22 3.02 3.32 LT-9 43.58 2.20 - LT-9 43.58 - 1.60 1.48 38.00 39.14 4.00 4.27 -
[1] 黄宝贵, 张志勇, 杨林, 等.中国化学物相分析研究的新成就 (上)[J].中国无机分析化学, 2011, 1(2):6-12. http://www.cnki.com.cn/Article/CJFDTOTAL-WJFX201102003.htm
Huang B G, Zhang Z Y, Yang L, et al.Recent achievements of chemical phase analysis in China (The First Volume)[J].Chinese Journal of Inorganic Analytical Chemistry, 2011, 1(2):6-12. http://www.cnki.com.cn/Article/CJFDTOTAL-WJFX201102003.htm
[2] 龚美菱.相态分析与地质找矿[M].北京:地质出版社, 2007.
Gong M L.Phase Analysis and Geological Prospecting[M].Beijing:Geological Publishing House, 2007.
[3] 王永磊, 陈毓川, 王登红, 等.中国锑矿主要矿集区及其资源潜力探讨[J].中国地质, 2013, 40(5):1366-1378. http://www.cnki.com.cn/Article/CJFDTOTAL-DIZI201305003.htm
Wang Y L, Chen Y C, Wang D H, et al.The principal antimony concentration areas in China and their resource potentials[J].Geology of China, 2013, 40(5):1366-1378. http://www.cnki.com.cn/Article/CJFDTOTAL-DIZI201305003.htm
[4] 岩石矿物分析编委会.岩石矿物分析 (第四版 第三分册)[M].北京:地质出版社, 2011:149-151.
The Editorial Committee of Rock and Mineral Analysis.Rock and Mineral Analysis (Fourth Edition:Vol.Ⅲ)[M].Beijing:Geological Publishing House, 2011:149-151.
[5] 黄宝贵, 吴爱华.高价态氧化锑的物质组成及价态分析[J].矿业工程, 1998, 18(4):50-53. http://www.cnki.com.cn/Article/CJFDTOTAL-KYGC804.014.htm
Huang B G, Wu A H.A method of valences analysis of Sb (Ⅲ, Ⅴ) in Sb6O13[J].Mineral Engineering, 1998, 18(4):50-53. http://www.cnki.com.cn/Article/CJFDTOTAL-KYGC804.014.htm
[6] 颜美英, 杨美云.锑品中不同价态氧化锑的分析方法[J].冶金分析, 1988, 8(5):31-34. http://www.cnki.com.cn/Article/CJFDTOTAL-YJFX198805011.htm
Yan M Y, Yang M Y.A analytical method for the determination of different valence[J].Metallurgical Analysis, 1988, 8(5):31-34. http://www.cnki.com.cn/Article/CJFDTOTAL-YJFX198805011.htm
[7] 陈家蓉.锑矿氯化浸出液中锑的价态分析[J].贵州工学院学报, 1994, 2(5):64-67. http://www.cnki.com.cn/Article/CJFDTOTAL-GZGX405.010.htm
Chen J Y.Analysis of valence conditions of atimony in chloridizing in each solution of stibnite[J].Journal of Guizhou Institute of Technlogy, 1994, 2(5):64-67. http://www.cnki.com.cn/Article/CJFDTOTAL-GZGX405.010.htm
[8] 阮鸿兴.化学物相分析过程中有关溶剂选择研究[J].云南冶金, 1994(1):56-60. http://www.cnki.com.cn/Article/CJFDTOTAL-YNYJ401.011.htm
Ruan H X.Study on the selection of solvents in the process of chemical phase analysis[J].Yunnan Metallurgy, 1994(1):56-60. http://www.cnki.com.cn/Article/CJFDTOTAL-YNYJ401.011.htm
[9] 郑森芳.原子吸收法测定锑矿中的氧化锑[J].分析试验室, 1987, 6(11):52. http://www.cnki.com.cn/Article/CJFDTOTAL-FXSY198711017.htm
Zheng S F.Determination of antimony oxide antimony in atomic absorption spectrometry[J].Analytical Laboratory, 1987, 6(11):52. http://www.cnki.com.cn/Article/CJFDTOTAL-FXSY198711017.htm
[10] 北京矿冶研究院.化学物相分析[M].北京:冶金工业出版社, 1979:42.
Beijing Research Institute of Mining and Metallurgy.Chemical Phase Analysis[M].Beijing:Metallurgical Industry Press, 1979:42.
-



 下载:
下载: