Influence Factors and Mechanism Study on Bag Sampling Method for Determination of Reduced Sulfide Compound by Gas Chromatography-Mass Spectrometry
-
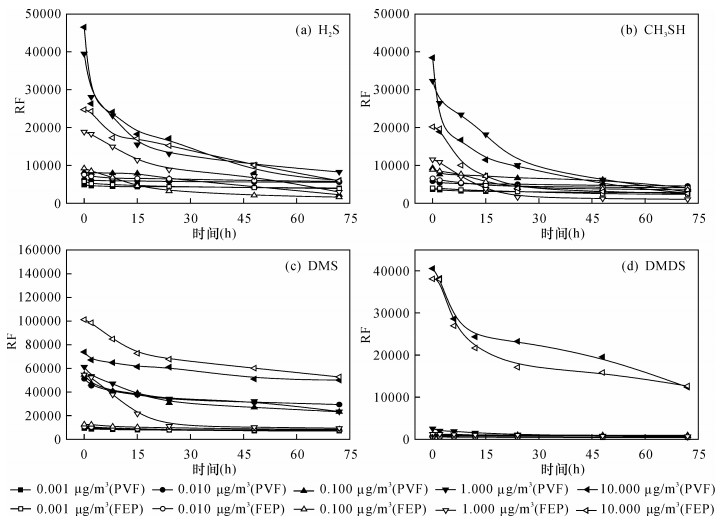
摘要: 还原硫化物是一Z类典型的恶臭物质,其特点是活性高、不易储存,因此适宜的储存条件对还原硫化物的准确测定具有重要意义。本文使用气相色谱-质谱联用技术,从气袋材质、还原硫化物初始浓度、还原硫化物性质和储存时间四个因素探究袋采样法储存还原硫化物过程的损失情况。以Tedlar®PVF和Teflon®FEP为目标采样袋,使用5个初始浓度(0.001、0.010、0.100、1.000和10.000μg/mL)的混合还原硫化物,选择0、2、6、12、24、48以及72h的储存时间,以响应因子和相对回收率作为评价因子,并使用配对t检验法和吸附动力学,研究影响储存效果的主要因素、物质损失机理以及两种采样袋的储存能力。结果表明,储存时间越长、物质初始浓度越高,物质活性越强,损失情况越严重;在环境温度达到60℃时,Tedlar®PVF的基质背景较Teflon®FEP更复杂;相同条件下,还原硫化物在Teflon®FEP储存过程中损失更严重。依据研究结果建议:①样品采集后避光保存;②低浓度含硫样品的测定在采样后8h内完成,高浓度含硫样品的测定在2h内完成;③若待测样品气体温度较高,优先选择Teflon®FEP采样袋,气体温度较低条件下选择Tedlar®PVF采样袋,可最大限度保持样品的原始状态。本研究成果有利于确保还原硫化物样品的储存稳定性,最大限度还原恶臭污染现场情况,为恶臭污染的分析测试以及后续的恶臭污染控制与治理提供技术支持。Abstract:
BACKGROUNDReduced sulfide compound is a typical odorant characterized by high activity and is difficult to store. Therefore, suitable storage conditions are of great significance for the accurate determination of reduced sulfides. OBJECTIVESTo understand the causes of loss and deterioration of highly active reduced sulfides, and to select the optimal storage conditions. METHODSGas Chromatography-Mass Spectrometry was used to investigate the loss of the process of storing reduced sulfides by bag sampling from the following four factors, air bag material, initial concentration of reduced sulfide, reduced sulfide property, and storage time. Sample bags, Tedlar®PVF and Teflon®FEP, were used as object bags. Using 5 initial concentrations (0.001, 0.010, 0.100, 1.000 and 10.000μg/mL) of mixed reduced sulfides, 0, 2, 6, 12, 24, 48 and 72h as the storage time, using the response factor and relative recovery as the evaluation factor, and using the paired t-test and adsorption kinetics, the main factors affecting the storage effect, the mechanism of material loss and the comparison of the storage capacity of the two sampling bags were studied. RESULTSThe results show that the longer the storage time, the higher the initial concentration of the substance, and the stronger the activity of the substance, the more serious the loss. When the ambient temperature reaches 60℃, the substrate background of Tedlar®PVF is more complicated than that of Teflon®FEP. Under the same conditions, the reduced sulfides are more severely damaged during Teflon®FEP storage. CONCLUSIONSIt was suggested that:(1) the reduced sulfide be stored in shad after sampling; (2) analysis should be finished within eight hours and two hours for low and high concentration, respectively; (3) Teflon®FEP be used at high temperature and Tedlar®PVF at low temperature. The research results were beneficial to ensure the storage stability of the reduced sulfide samples, minimize the situation of odor pollution, and provide technical support for the analysis of odor pollution and the subsequent control and treatment of odor pollution. -

-
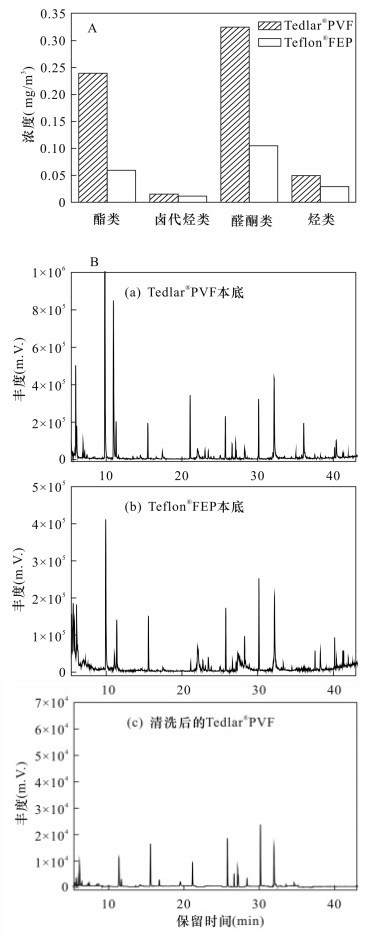
表 1 两种采样袋内本底物质及浓度
Table 1. Sample bags substrate compound and concentration
物质名称 浓度(mg/m3) TedlarⓇPVF TedlarⓇFEP 乙酸乙酯 0.1695 0.0277 乙酸异丙酯 0.0221 n.d. 乙酸正丙酯 0.0168 n.d. 乙酸正丁酯 0.0311 0.0316 氯甲烷 0.0019 n.d. 三氯氟甲烷 0.0046 0.0046 氯仿 0.0035 0.0027 1, 2-二氯乙烷 0.0049 0.0040 乙醇 0.1210 0.0188 丙酮 0.0212 0.0114 2-丁酮 0.1829 0.0745 总挥发性
有机物1.2594 0.4096 丙烯 0.0025 n.d. 丙烷 0.0028 n.d. 苯 0.0029 0.0025 甲苯 0.0156 0.0051 乙苯 0.0017 0.0008 间二甲苯 0.0060 0.0048 对二甲苯 0.0046 0.0039 苯乙烯 0.0042 0.0034 邻二甲苯 0.0042 0.0039 1, 2, 4-
三甲苯0.0047 0.0047 对二乙苯 0.0004 n.d. 苯酚* 0.0012 0.0008 注:“*”表示以甲苯计的半定量结果;n.d.表示未检出。 表 2 不同浓度还原硫化物在TedlarⓇPVF和TedlarⓇFEP采样袋储存72h后的相对回收率及平均值和标准偏差、P值和t值
Table 2. Relative recovery, mean, standard deviation, P value and t value for each individual RSC in TedlarⓇPVF and TedlarⓇFEP
还原硫化物浓度
(μg/mL)H2S回收率(%) CH3SH回收率(%) DMS回收率(%) DMDS回收率(%) TedlarⓇPVF TedlarⓇFEP TedlarⓇPVF TedlarⓇFEP TedlarⓇPVF TedlarⓇFEP TedlarⓇPVF TedlarⓇFEP 0.001 82.88 67.03 76.75 57.76 76.51 72.41 73.31 80.59 0.010 89.40 76.35 78.24 62.11 57.66 70.82 68.50 69.91 0.100 27.78 17.62 43.62 39.64 42.72 66.34 82.64 85.50 1.000 21.01 15.78 9.15 8.54 38.37 17.24 21.21 55.51 10.000 13.00 24.06 6.80 12.42 67.75 52.15 30.43 33.17 平均值(%) 46.81 40.17 42.91 36.09 56.60 55.79 55.22 64.94 标准偏差(%) 36.35 29.13 34.78 24.89 16.18 22.98 27.51 21.16 P值 0.2353 0.2181 0.9282 0.1932 t值 1.40 1.46 0.10 1.56 表 3 采样袋内壁吸附不同浓度还原硫化物的一阶动力学常数和半衰期
Table 3. Summary of the first-order loss coefficient and half-life time for each individual RSC in varying concentration levels
还原硫化物 采样袋材质 浓度
(mg/m3)k1(h-1) t1/2(h) H2S TedlarⓇPVF 0.001 0.0069 100.46 0.010 0.0069 100.46 0.100 0.0117 59.24 1.000 0.0122 56.82 10.000 0.0139 49.87 TedlarⓇFEP 0.001 0.008 86.64 0.010 0.0078 88.87 0.100 0.0211 62.45 1.000 0.0249 27.84 10.000 0.0235 29.50 CH3SH TedlarⓇPVF 0.001 0.0095 72.96 0.010 0.0069 100.46 0.100 0.0104 66.65 1.000 0.0227 30.54 10.000 0.0158 43.87 TedlarⓇFEP 0.001 0.0184 37.67 0.010 0.0097 71.46 0.100 0.0179 38.72 1.000 0.0253 27.40 10.000 0.0243 28.52 DMS TedlarⓇPVF 0.001 0.0086 80.60 0.010 0.0089 77.88 0.100 0.0116 59.75 1.000 0.0122 56.82 10.000 0.0195 35.55 TedlarⓇFEP 0.001 0.0093 74.53 0.010 0.0113 61.34 0.100 0.0162 42.79 1.000 0.0222 31.22 10.000 0.0159 43.59 DMDS TedlarⓇPVF 0.001 0.0043 161.20 0.010 0.0097 71.46 0.100 0.0129 53.73 1.000 0.0161 43.05 10.000 0.0163 42.52 TedlarⓇFEP 0.001 0.0125 55.45 0.010 0.0228 30.40 0.100 0.0145 47.08 1.000 0.0299 23.18 10.000 0.0249 27.84 -
[1] Almomani F A, Bhosale R R, Kumar A, et al.Removal of volatile sulfur compounds by solar advanced oxidation technologies and bioprocesses[J].Solar Energy, 2016, 135:348-358. doi: 10.1016/j.solener.2016.05.037
[2] Nagata Y.Measurement of Odor Threshold by Triangle Odor Bag Method[R].Odor Measurement Review, Ministry of Environment (MOE), Japan, 2003: 118-127.
[3] Li Q, Lancaster Jr J R.Chemical foundations of hydrogen sulfide biology[J].Nitric Oxide, 2013, 35:21-34. doi: 10.1016/j.niox.2013.07.001
[4] 沈秀娥, 常淼, 刘保献, 等.大气预浓缩仪-GC/FPD测定环境空气中的痕量硫化物[J].中国环境监测, 2015, 31(6):103-108. doi: 10.3969/j.issn.1002-6002.2015.06.022
Shen X E, Chang M, Liu B X, et al.Determination of trace sulfur compounds in environmental air by GC/FPD coupled the air preconcentrations[J].Environmental Monitoring in China, 2015, 31(6):103-108. doi: 10.3969/j.issn.1002-6002.2015.06.022
[5] 孟天竹, 张金波, 蔡祖聪.气相色谱法测定土壤中挥发性硫化物[J].环境监测管理与技术, 2016, 28(1):46-49. doi: 10.3969/j.issn.1006-2009.2016.01.012
Meng T Z, Zhang J B, Cai Z C.Determination of sulfur gases emitted from soils by gas chromatography with sulfur chemiluminescence detector[J].The Administration and Technique of Environmental Monitoring, 2016, 28(1):46-49. doi: 10.3969/j.issn.1006-2009.2016.01.012
[6] Borrás E, Tortajada-Genaro L A, Muñoz A.Determina-tion of reduced sulfur compounds in air samples for the monitoring of malodor caused by landfills[J].Talanta, 2016, 148:472-477. doi: 10.1016/j.talanta.2015.11.021
[7] Sulyok M, Haberhauer-Troyer C, Rosenberg E.Obser-vation of sorptive losses of volatile sulfur compounds during natural gas sampling[J].Journal of Chromatography A, 2002, 946(1-2):301-305. doi: 10.1016/S0021-9673(01)01541-2
[8] Trabue S, Scoggin K, Mitloehner F, et al.Field sampling method for quantifying volatile sulfur compounds from animal feeding operations[J].Atmospheric Environment, 2008, 42(14):3332-3341. doi: 10.1016/j.atmosenv.2007.03.016
[9] 施玉格, 李刚.气相色谱质谱法测定环境空气中恶臭硫化物成分[J].干旱环境监测, 2014, 28(4):174-177. doi: 10.3969/j.issn.1007-1504.2014.04.007
Shi Y G, Li G.Analysis of the stench sulfide components in the environmental air by GC/MS[J].Arid Environmental Monitoring, 2014, 28(4):174-177. doi: 10.3969/j.issn.1007-1504.2014.04.007
[10] Parker D B, Perschbacher-Buser Z L, Andy C N, et al. Recovery of agricultural odors and odorous compounds from polyvinyl fluoride film bags[J].Sensors, 2010, 10(9):8536-8552. doi: 10.3390/s100908536
[11] Mochalski P, Wzorek B, Śliwka I, et al.Suitability of different polymer bags for storage of volatile sulphur compounds relevant to breath analysis[J].Journal of Chromatography B, 2009, 877(3):189-196. doi: 10.1016/j.jchromb.2008.12.003
[12] Jo S H, Kim K H, Shon Z H, et al.Identification of con-trol parameters for the sulfur gas storability with bag sampling methods[J].Analytica Chimica Acta, 2012, 738:51-58. doi: 10.1016/j.aca.2012.06.010
[13] Kim Y H, Kim K H, Jo S H, et al.Comparison of storage stability of odorous VOCs in polyester aluminum and polyvinyl fluoride Tedlar® bags[J].Analytica Chimica Acta, 2012, 712:162-167. doi: 10.1016/j.aca.2011.11.014
[14] Ahn J H, Deep A, Kim K H.The storage stability of bio-genic volatile organic compounds (BVOCs) in polyester aluminum bags[J].Atmospheric Environment, 2016, 14:430-434. http://cn.bing.com/academic/profile?id=bff720658888ab9c866b77990c154886&encoded=0&v=paper_preview&mkt=zh-cn
[15] 董秀玥.配对t检验与成组t检验优选方法研究[J].数理医药学杂志, 2010, 23(1):11-14. doi: 10.3969/j.issn.1004-4337.2010.01.004
Dong X Y.Study on the optimal method of paired t test and group t test[J].Journal of Mathematical Medicine, 2010, 23(1):11-14. doi: 10.3969/j.issn.1004-4337.2010.01.004
[16] Sulyok M, Haberhauer-Troyer C, Rosenberg E.Investiga-tion of the stability of selected volatile sulfur compounds in different sampling containers[J].Journal of Chromatography A, 2001, 917(1-2):367-374. doi: 10.1016/S0021-9673(01)00654-9
[17] 武汉大学.分析化学(第四版)[M].北京:高等教育出版社, 2000.
Wuhan University.Analytical Chemistry(4th Edition)[M].Beijing:Higher Education Press, 2000.
[18] Foo K Y, Hameed B H.Insights into modeling of adsor-ption isotherm systems[J].Chemical Engineering Journal, 2010, 156(1):2-10. doi: 10.1016/j.cej.2009.09.013
[19] Andino J M, Bulter J W.A study of stability of method-fueled vehicle emission in tedlar bags[J].Environment Science Technology, 1991, 25(9):1644-1649. doi: 10.1021/es00021a017
[20] Behera S N, Sharma M.Degradation of SO2, NO2 and NH3 leading to formation of secondary inorganic aerosols:An environmental chamber study[J].Atmospheric Environment, 2011, 45(24):4015-4024. doi: 10.1016/j.atmosenv.2011.04.056
-



 下载:
下载: