Determination of Fe(Ⅱ) and Total Iron in Red Sandstone by o-phenanthroline Spectrophotometry
-
摘要: 红层砂岩是丹霞地貌区的一套陆相或浅水湖相沉积碎屑岩,准确分析红层砂岩中Fe(Ⅱ)和Fe(Ⅲ)在风化过程中的氧化还原关系,可作为该岩块风化程度以及深度的划分依据。在应用邻菲啰啉分光光度法测定铁含量过程中,Fe(Ⅱ)极易被氧化而导致结果偏低,有效防止氧化作用是准确测量Fe(Ⅱ)和Fe(Ⅲ)含量的重点。本文利用Fe(Ⅱ)与邻菲啰啉形成有色络合物的特点,在红层砂岩样品中滴加适量显色剂后用氢氟酸-稀硫酸溶解,取同一溶清液分别经过盐酸羟胺还原、邻菲啰啉显色、乙酸-乙酸铵缓冲液调节pH等步骤后,在分光光度计下测定微量Fe(Ⅱ)和全铁含量。该分析方法测定Fe(Ⅱ)的检出限为0.002%,RSD(n=8)小于2%,加标回收率为92.6%~94.7%。利用该分析方法测定赤水丹霞地貌区红层砂岩中Fe(Ⅱ)含量在0.01%~0.1%,全铁为0.7%~1.5%,差减法计算得Fe(Ⅲ)含量在0.7%~1.5%,测定结果与X射线荧光光谱法、重络酸钾滴定法一致。本方法采用显色剂和氢氟酸-稀硫酸溶解,能有效保存砂岩中原生Fe(Ⅱ),使用同一溶清液既能测定全铁,也能准确测定Fe(Ⅱ),为分析红层砂岩中各类铁含量提供了一种简单、准确的分析方法。Abstract:
BACKGROUNDRed bed sandstone is a set of continental or shallow lake clastic rocks in the Danxia landform. Accurate determination of Fe(Ⅱ) and Fe(Ⅲ) in red bed sandstone that indicates the redox relationship during weathering can serve as the dividing basis of weathering degree and depth of the block. During the determination of iron content by phenanthroline spectrophotometry, Fe(Ⅱ) is easily oxidized and the result is a lower value than it should be. Effective prevention of oxidation is the focus of accurate measurement of Fe(Ⅱ) and Fe(Ⅲ) content. OBJECTIVESTo explore the preservation method of Fe(Ⅱ) during dissolution and establish a spectrophotometric method for the determination of Fe(Ⅱ) and total iron in red sandstone. METHODSColor reagents were added in the samples, and the samples were dissolved by hydrofluoric acid and dilute sulfuric acid, in a constant temperature water bath at 90℃. Hydroxylamine hydrochloride was used as a reducing agent, o-phenanthroline was used as a chromogenic agent, pH was adjusted by acetate-ammonium acetate buffer, and trace Fe(Ⅱ) and total iron were determined by spectrophotometer. RESULTSThe detection limit of Fe(Ⅱ) was 0.002%, relative standard deviation (RSD, n=8) was less than 2%, and the standard recovery was between 92.6% and 94.7%. The Fe(Ⅱ) content of red sandstone in the Danxia landform area of Chishui was determined by this analysis method. The content of Fe(Ⅱ) ranged from 0.01% to 0.1%, the total iron content was 0.7%-1.5%, and the content of Fe(Ⅲ) calculated by subtraction was 0.7%-1.5%. The measurement results were consistent with those of X-ray fluorescence spectrometry and potassium complexate titration method. CONCLUSIONSThis method uses developer and hydrofluoric acid-dilute sulfuric acid to dissolve the samples, which can effectively preserve the original Fe(Ⅱ) in sandstone. The same solution can be used for the determination of both total iron and Fe(Ⅱ) which provides a simple and accurate analysis method for various types of iron content in red bed sandstone. -
Key words:
- red sandstone /
- Fe (Ⅱ) /
- total iron /
- acid dissolution /
- spectrophotometry /
- o-phenanthroline
-

-
表 1 显色液中显色剂用量分析
Table 1. Analysis of the color reagent dosage in color solution
显色剂用量(mL) Fe(Ⅱ)吸光度 全铁吸光度 Y1 Y2 Y3 Y1 Y2 Y3 0.0 0.086 0.165 0.361 0.182 0.174 0.205 0.2 0.099 0.187 0.399 0.643 0.548 0.543 0.4 0.102 0.184 0.415 0.642 0.540 0.554 0.6 0.098 0.188 0.413 0.650 0.539 0.550 0.8 0.101 0.186 0.415 0.648 0.546 0.556 1.0 0.102 0.188 0.414 0.640 0.541 0.553 表 2 空白溶液的吸光值与其对应的浓度值
Table 2. Absorbance value of blank solution and its corresponding concentration value
参数 检出值 平均值 标准偏差 吸光度 0.075 0.074 0.076 0.074 0.076 0.075 0.076 0.073 0.075 0.074 0.0745 0.0011 0.076 0.075 0.075 0.073 0.074 0.074 0.073 0.073 0.074 0.075 浓度(mg/L) 0.3463 0.3414 0.3511 0.3414 0.3511 0.3463 0.3511 0.3366 0.3463 0.3414 0.3438 0.0051 0.3511 0.3463 0.3463 0.3366 0.3414 0.3414 0.3366 0.3366 0.3414 0.3463 表 3 精密度实验
Table 3. Experimental precision analysis
样品编号 检测项目 吸光度测定值 吸光度平均值 质量分数(%) RSD(%) 样品1 Fe(Ⅱ) 0.838 0.85 0.847 0.867 0.8536 0.27 1.56 0.856 0.832 0.857 0.882 全铁 0.595 0.578 0.614 0.600 0.5985 1.13 1.21 0.611 0.596 0.606 0.588 样品2 Fe(Ⅱ) 0.126 0.137 0.13 0.145 0.1345 0.25 0.56 0.134 0.137 0.133 0.134 全铁 0.407 0.418 0.41 0.417 0.4134 0.78 0.44 0.413 0.409 0.419 0.414 表 4 测定微量Fe(Ⅱ)分析方法的加标回收率数据
Table 4. Data of standard addition recovery for determination of trace Fe(Ⅱ) analysis
样品编号 试样加入量(mg) Fe(Ⅱ)标准液加入量(mL) 计算值(mg) 测定值(mg) 回收率(%) 平均值(%) 样品1 0 0.5
1
1.50.05
0.1
0.150.0488
0.0951
0.139297.6
95.1
92.895.2 50 0
0.5
1
1.5/
0.1936
0.2436
0.29360.1436
0.1908
0.2427
0.2799/
94.3
99.1
90.894.7 样品2 0 0.5
1
1.50.01
0.02
0.030.0097
0.0209
0.03096.7
104.4
102.9101.4 50 0
0.5
1
1.5/
0.0388
0.0488
0.05880.0288
0.0384
0.0468
0.0563/
95.9
90.2
91.592.6 表 5 不同测定方法的数据分析
Table 5. Data analysis of different determination methods
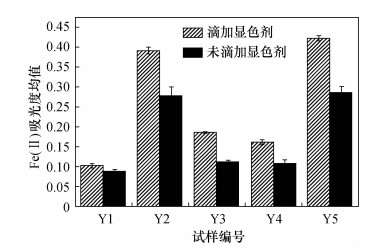
样品编号 Fe(Ⅱ)测定值(%) 重络酸钾容量法 邻菲啰啉分光光度法 Y-1 0.1089 0.1082 Y-2 0.0544 0.0558 Y-3 0.1089 0.1146 Y-4 0.0389 0.0435 Y-5 0.0467 0.0476 -
[1] 朱诚.对用Fe3+/F2+探讨庐山地区第四纪古温度的讨论[J].地质论评, 1994(3):216-220.
Zhu C.The quaternary paleotemperature in Lushan area is discussed by Fe3+/F2+[J]. Geological Review, 1994(3):216- 220.
[2] 迟振卿, 闵隆瑞, 武志军.河北阳原盆地井儿洼钻孔岩心氧化铁变化的古环境记录[J].地质通报, 2002, 21(10):632-637. http://d.old.wanfangdata.com.cn/Periodical/zgqydz200210006
Chi Z Q, Min L R, Wu Z J.Paleoenvironmental records of changes in core iron oxide in Jingerwa, Yangyuan Basin, Hebei Province[J]. Geological Bulletin of China, 2002, 21(10):632-637. http://d.old.wanfangdata.com.cn/Periodical/zgqydz200210006
[3] 张飞飞, 彭乾云, 朱祥坤, 等.湖北古城锰矿Fe同位素特征及其古环境意义[J].地质学报, 2013, 87(9):1411-1418. http://d.old.wanfangdata.com.cn/Periodical/dizhixb201309017
Zhang F F, Peng Q Y, Zhu X K, et al.Fe isotopic characteristics and paleoenvironmental significance of Gucheng manganese mine, Hubei Province[J]. Acta Geologica Sinica, 2013, 87(9):1411-1418. http://d.old.wanfangdata.com.cn/Periodical/dizhixb201309017
[4] 肖育雄, 黎晏彰, 丁竑瑞, 等.湖南新宁崀山丹霞红层天然半导体矿物的矿物学特征研究[J/OL].北京大学学报(自然科学版): 1-11[2019-08-12].https://doi.org/10.13209/j.0479-8023.2019.065.
Xiao Y X, Li Y Z, Ding X R, et al.Mineralogical characteristics of natural semiconductor minerals in Danxia red layer, Langshan, Xinning, Hunan[J/OL]. Acta Scientiarum Naturalium Universitatis Pekinensis: 1-11[2019-08-12]. https://doi.org/10.13209/j.0479-8023.2019.065.
[5] 苏洋.邻菲罗啉光度法测定钒铝合金中铁[J].冶金分析, 2019, 39(2):77-81. http://d.old.wanfangdata.com.cn/Periodical/yjfx201902014
Su Y.Determination of iron in vanadium aluminum alloy by o-phenanthroline spectrophotometry[J]. Metallurgical Analysis, 2019, 39(2):77-81. http://d.old.wanfangdata.com.cn/Periodical/yjfx201902014
[6] 陈建津, 陈嘉敏, 张志强, 等.邻菲啰啉分光光度法测定白砂糖中铁含量[J].现代食品, 2016(12):79-81. http://d.old.wanfangdata.com.cn/Periodical/lsltjs201612031
Chen J J, Chen J M, Zhang Z Q, et al.Determination of iron in sugar by o-phenanthroline spectrophotometry[J]. Modern Food, 2016(12):79-81. http://d.old.wanfangdata.com.cn/Periodical/lsltjs201612031
[7] 祁极冰, 刘有智, 罗莹, 等.邻菲啰啉分光光度法测定络合铁脱硫液中铁的含量[J].现代化工, 2016, 36(1):187-190. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201601046
Qi J B, Liu Y Z, Luo Y, et al.Determination of iron in complex iron desulfurization solution by o-phenanthroline spectrophotometry[J]. Modern Chemical Industry, 2016, 36(1):187-190. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdhg201601046
[8] 徐勋达, 胡璐, 余国贤, 等.改进邻菲啰啉分光光度法测定络合铁中亚铁含量[J].江汉大学学报(自然科学版), 2016, 44(1):48-51.
Xu X D, Hu L, Yu G X, et al.Improving the spectrophotometry for the determination of iron content in Central Asia with complex iron[J]. Journal of Jianghan University (Natural Science Edition), 2016, 44(1):48-51.
[9] 尹辉, 施鸿金, 龙春月, 等.邻二氮菲分光光度法测定几种日常食物中的铁含量[J].科教导刊, 2015(3):36. http://d.old.wanfangdata.com.cn/Periodical/kjdk201508018
Yin H, Shi H J, Long C Y, et al.Determination of iron in several daily foods by phenanthrene spectrophotometry[J]. The Guide of Science and Education, 2015(3):36. http://d.old.wanfangdata.com.cn/Periodical/kjdk201508018
[10] 傅翠梨, 陈文瑞, 郭阿明, 等.邻菲啰啉光度法测定高岭土中可溶铁和非可溶铁[J].岩矿测试, 2012, 31(4):621-626. http://www.ykcs.ac.cn/article/id/ykcs_20120412
Fu C L, Chen W R, Guo A M, et al.Determination of soluble iron and non-soluble iron in kaolin by o-phenanthroline spectrophotometry[J]. Rock and Mineral Analysis, 2012, 31(4):621-626. http://www.ykcs.ac.cn/article/id/ykcs_20120412
[11] 韩林宝, 代群威, 党政, 等.厌氧环境下邻菲罗啉分光光度法测定纤维水镁石中Fe(Ⅱ)与Fe(Ⅲ)[J].冶金分析, 2018, 38(5):25-29. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201805005
Han L B, Dai Q W, Dang Z, et al.Determination of Fe(Ⅱ) and Fe(Ⅲ) in fiber brucite by o-phenanthroline spectrophotometry in anaerobically environment[J]. Metallurgical Analysis, 2018, 38(5):25-29. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201805005
[12] 吴永明, 陶武, 黄飞雪, 等.邻菲罗啉示差分光光度法测定铁矿石中全铁含量[J].化学试剂, 2018, 40(1):45-48. http://d.old.wanfangdata.com.cn/Periodical/huaxsj201801009
Wu Y M, Tao W, Huang F X, et al.Determination of total iron in iron ore by o-phenanthroline differential spectrophotometry[J]. Chemical Reagents, 2018, 40(1):45-48. http://d.old.wanfangdata.com.cn/Periodical/huaxsj201801009
[13] 李令, 朱云勤, 张菊, 等.邻二氮菲分光光度法测定赤泥和石灰石混匀度[J].岩矿测试, 2009, 28(4):394-396. http://www.ykcs.ac.cn/article/id/ykcs_20090422
Li L, Zhu Y Q, Zhang J, et al.Determination of mixing degree of red mud and limestone by phenanthrene spectrophotometry[J]. Rock and Mineral Analysis, 2009, 28(4):394-396. http://www.ykcs.ac.cn/article/id/ykcs_20090422
[14] 冉广芬, 马海州.分光光度法同时测定岩盐中微量Fe2+和Fe3+的研究[J].盐湖研究, 2008(1):22-26. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yhyj200801005
Ran G F, Ma H Z.Simultaneous determination of trace Fe2+ and Fe3+ in rock salt by spectrophotometry[J]. Journal of Salt Lake Research, 2008(1):22-26. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yhyj200801005
[15] 潘涵香, 胡超涌, 王红梅, 等.邻二氮菲分光光度法测定碳酸盐相中微量亚铁[J].岩矿测试, 2007, 26(3):198-200. http://www.ykcs.ac.cn/article/id/ykcs_20070369
Pan H X, Hu C Y, Wang H M, et al.Determination of trace ferrous in carbonate phase by o-phenanthrene spectrophotometry[J]. Rock and Mineral Analysis, 2007, 26(3):198-200. http://www.ykcs.ac.cn/article/id/ykcs_20070369
[16] 姚进一, 白晓龙, 花海蓉, 等.如何提高分光光度分析的准确度[J].中国环境监测, 2017, 33(2):116-121. http://d.old.wanfangdata.com.cn/Periodical/zghjjc201702018
Yao J Y, Bai X L, Hua H R, et al.How to improve the accuracy of spectrophotometry[J]. Environmental Monitoring in China, 2017, 33(2):116-121. http://d.old.wanfangdata.com.cn/Periodical/zghjjc201702018
[17] Alexandre S.Anastácio, Harris B, Yoo H I, et al. Limitations of the ferrozine method for quantitative assay of mineral systems for ferrous and total iron[J]. Geochimica et Cosmochimica Acta, 2008, 72(20):0-5008. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1b83673f94769d8b5d34b482c726c46c
[18] Remucal C K, Sedlak D L.The role of iron coordination in the production of reactive oxidants from ferrous iron oxidation by oxygen and hydrogen peroxide[J]. ACS Symposium Series, 2011, 1071:177-197.
[19] 武汉大学.分析化学(第四版)[M].北京:高等教育出版社, 2000.
Wuhan University.Analytical Chemistry (The Fourth Edition)[M]. Beijing:Higher Education Press, 2000.
[20] Satyanarayanan M, Balaram V, Sawant S S, et al.Rapid determination of REEs, PGEs, and other trace elements in geological and environmental materials by high resolution inductively coupled plasma mass spectrometry[J]. Atomic Spectroscopy, 2018, 39(1):1-15. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=399d63251e1afd27679392d6e00fdd4d
[21] Yin X, Wang X, Chen S, et al.Trace element determination in sulfur samples using a novel digestion bomb prior to ICP-MS analysis[J]. Atomic Spectroscopy, 2018, 39(4):137-141. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fc0b26c03318c4a8d7120f80ec5663cc
[22] Oral E V.Comparison of two sequential extraction procedures for trace metal partitioning in ore samples from the Keban Region in Elazig, Turkey[J]. Atomic Spectroscopy, 2018, 39(5):198-202.
[23] 温建淳.在高铁背景下二价铁含量的测定方法浅析[J].科技创新导报, 2017, 14(30):103-107. http://d.old.wanfangdata.com.cn/Periodical/kjzxdb201730057
Wen J C.Analysis on the determination method of divalent iron content under the background of high iron[J]. Science and Technology Innovation Herald, 2017, 14(30):103-107. http://d.old.wanfangdata.com.cn/Periodical/kjzxdb201730057
[24] Tamura H, Goto K, Yotsuyanagi T, et al.Spectro- photometric determination of iron(Ⅱ) with 1, 10-phenanthroline in the presence of large amounts of iron(Ⅲ)[J]. Talanta, 1974, 21(4):314-318.
[25] 张会堂.邻菲啰啉助溶保护-容量法测定电气石中的氧化亚铁[J].山东国土资源, 2018, 34(6):80-83. http://d.old.wanfangdata.com.cn/Periodical/sddz201806013
Zhang H T.Determination of the ferrous oxide in the tourmaline by the solubility protection-capacity method of the o-phenanthrene[J]. Shandong Land and Resources, 2018, 34(6):80-83. http://d.old.wanfangdata.com.cn/Periodical/sddz201806013
[26] 杨朋兵, 林兴, 李祥, 等.邻菲罗啉分光光度法测定含EDTA溶液中Fe3+ 的改进[J].实验室研究与探索, 2016, 35(12):22-25. http://d.old.wanfangdata.com.cn/Periodical/sysyjyts201612006
Yang P B, Lin X, Li X, et al.Improvement in determination of Fe3+ in EDTA-containing solution by o-phenanthroline spectrophotometry[J]. Research and Exploration of Laboratory, 2016, 35(12):22-25. http://d.old.wanfangdata.com.cn/Periodical/sysyjyts201612006
[27] 曾子琦, 李欣, 李跑.邻二氮菲比色法测定铁含量的条件优化研究[J].广州化工, 2018, 46(14):77-80. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gzhg201814030
Zeng Z Q, Li X, Li P.Study on the optimization of the conditions for the determination of iron content by o-phenanthrene colorimetric method[J]. Guangzhou Chemical Industry, 2018, 46(14):77-80. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gzhg201814030
[28] 姚雪霞, 戴存礼, 李晓林, 等.邻菲罗啉分光光度法测定微量铁实验条件的探讨[J].实验室研究与探索, 2015, 34(3):16-19. http://d.old.wanfangdata.com.cn/Periodical/sysyjyts201503005
Yao X X, Dai C L, Li X L, et al.Study on the experimental conditions for the determination of trace iron by o-phenanthroline spectrophotometry[J]. Research and Exploration of Laboratory, 2015, 34(3):16-19. http://d.old.wanfangdata.com.cn/Periodical/sysyjyts201503005
[29] 陈爽, 徐接胜.关于检出限的定义、分类及估算方法的探讨[J].广州化工, 2014, 42(18):137-139. http://d.old.wanfangdata.com.cn/Periodical/gzhg201418052
Chen S, Xu J S.On the definition, classification and estimation of detection limit[J]. Guangzhou Chemical Industry, 2014, 42(18):137-139. http://d.old.wanfangdata.com.cn/Periodical/gzhg201418052
[30] 伊郎, 宋盼盼, 刘宏.检出限选择性计算方法研究[J].计量与测试技术, 2015, 42(12):50-51. http://d.old.wanfangdata.com.cn/Periodical/jlycsjs201512022
Yi L, Song P P, Liu H.Study on the calculation method of detection limit selectivity[J]. Metrology and Measurement Techniques, 2015, 42(12):50-51. http://d.old.wanfangdata.com.cn/Periodical/jlycsjs201512022
-



 下载:
下载: