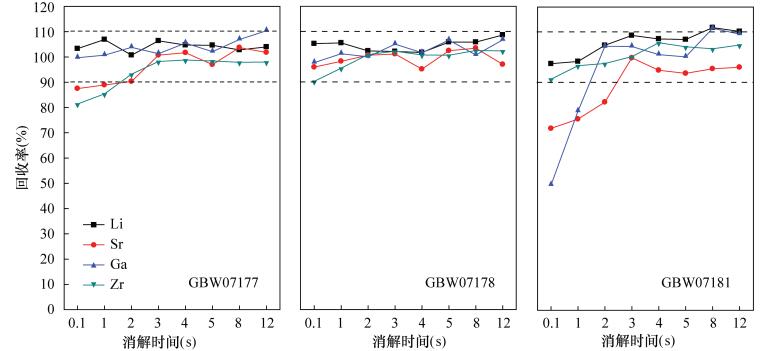
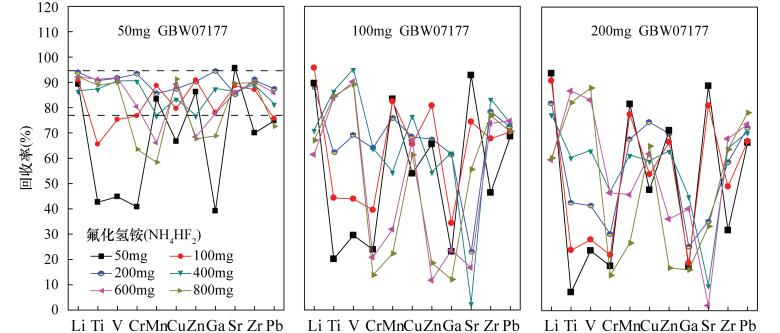
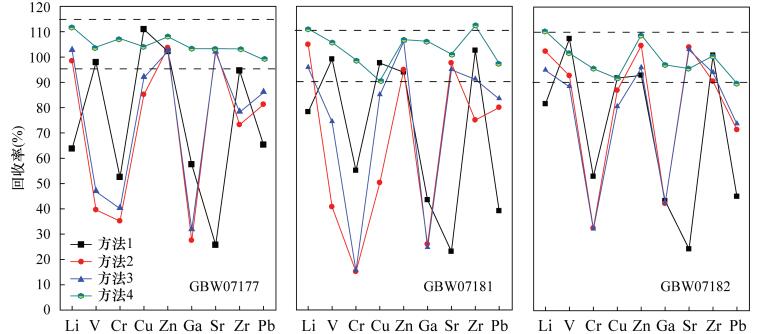
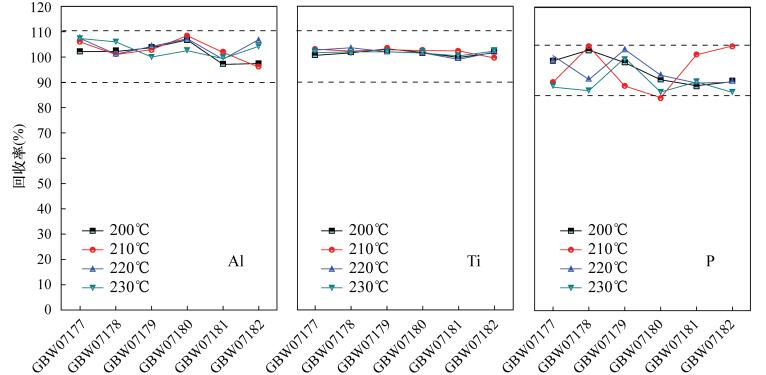
Determination of Lithium, Gallium, Zirconium, Rare Earth Elements and Other Trace Elements in Corundum-bearing Bauxite by Inductively Coupled Plasma-Mass Spectrometry with Rapid Decomposition of Ammonium Bifluoride
-
摘要: 铝土矿中常伴有锂、镓、锆、稀土等有用组分,完全提取并准确测定其含量对于铝土矿资源综合评价和综合利用具有重要意义。然而铝土矿中常常含有少量刚玉,常规的四酸、五酸和封闭压力酸溶法对其分解不完全,导致测定结果偏低。本文采用氟化氢铵作熔剂,高温下在旋盖PFA小瓶中分解样品,通过在熔样过程中使用少量硫酸,对不同熔矿温度、消解时间及试剂用量等因素详细考察,确定了最佳熔矿条件[200℃,3h,试样比4:1(称样量50mg)],建立了氟化氢铵分解-电感耦合等离子体质谱法测定含刚玉铝土矿中37种痕量元素的分析方法。本方法能快速、有效地分解含刚玉铝土矿,经三种铝土矿国家标准物质GBW07177、GBW07181和GBW07182验证,并与四酸、五酸和封闭压力酸溶法的测定结果对比,三种标准物质中Li、Ga、Sr、Zr、Pb等9种元素的回收率分别在95.0%~115.0%、90.0%~110.0%和90.0%~110.0%之间,测定值与认定值相符。同时,本方法实现了铝土矿(Al2O3含量在42.97%~90.36%之间)中Al、Ti、P等主量元素的精确分析,进一步验证了其用来测定铝土矿中痕量元素的准确性。方法检出限为0.002~0.43μg/g,与传统硝酸-氢氟酸密闭消解法的检出限(0.000~0.48μg/g)基本相当,精密度在1.14%~8.84%之间,能够满足铝土矿中痕量元素的分析要求。
-
关键词:
- 氟化氢铵 /
- 快速分解 /
- 电感耦合等离子体质谱法 /
- 含刚玉铝土矿 /
- 痕量元素
Abstract:BACKGROUND Bauxite is often accompanied by useful components such as lithium, gallium, zirconium, and rare earth metals. Complete extraction and accurate determination of the content of these components are of great significance for the comprehensive evaluation and comprehensive utilization of bauxite resources. However, bauxite often contains a small amount of corundum, which is not completely decomposed by the conventional four-acid, five-acid and closure pressure acid dissolution methods, resulting in lower measurement results. OBJECTIVES To explore the new decomposition method to achieve rapid and accurate analysis of trace elements in corundum- bearing bauxite. METHODS A digestion technique using the solid compound ammonium bifluoride in a screw-capped PFA vial at high temperature has been developed for trace elements analysis of corundum-bearing bauxite by using a small amount of sulfuric acid during the fusion process. The factors such as different melting temperature, digestion time and reagent dosage were investigated in detail, the optimal smelting conditions (200℃, 3h, sample ratio 4:1) were confirmed. An analytical method for determination of 37 trace elements in corundum-bearing bauxite by inductively coupled plasma-mass spectrometry with rapid decomposition of ammonium bifluoride was established. RESULTS This method can be used to quickly and effectively decompose corundum-bearing bauxite, which has been verified by three national bauxite standard materials GBW07177, GBW07181 and GBW07182. The proposed method was also compared with the results of the four-acid, five-acid and closure pressure acid dissolution methods. The recoveries of nine elements such as Li, Ga, Sr, Zr and Pb in the three standard materials were from 95.0% to 115.0%, 90.0% to 110.0%, and 90.0% to 110.0%, respectively. The analytical result was in agreement with the certified values. The detection limits of the method were from 0.002 to 0.43μg/g, which was closely equivalent to the detection limits (0.000-0.48μg/g) of the traditional nitric acid-hydrofluoric acid closure digestion method. The precisions were from 1.14% to 8.84%, which qualified it to meet the analytical requirements of trace elements in bauxite. CONCLUSIONS This method can be used to achieve accurate analysis of major elements such as Al, Ti, and P in bauxite (Al2O3 content between 42.97% and 90.36%), which further verifies the accuracy of this method for the determination of trace elements in bauxite. -

-
表 1 四种溶(熔)矿方法下铝土矿标准物质(GBW07177、GBW07181、GBW07182)中37种痕量元素的ICP-MS分析结果
Table 1. ICP-MS analytical results for determination of 37 trace elements in bauxite standard materials (GBW07177, GBW07181, GBW07182) by four sample dissolution (melting) methods
待测
元素认定值(μg/g) 方法一(μg/g) 方法二(μg/g) 方法三(μg/g) 方法四(μg/g) GBW
07177GBW
07181GBW
07182GBW
07177GBW
07181GBW
07182GBW
07177GBW
07181GBW
07182GBW
07177GBW
07181GBW
07182GBW
07177GBW
07181GBW
07182Li 80.6±5.5 35.1±4 147±12 51.3 27.5 120 79.3 36.8 151 82.6 33.6 140 90.2 39.0 162 Be / / / 7.20 5.43 5.18 3.78 3.81 3.71 4.25 3.33 3.60 8.86 6.83 5.52 Sc / / / 6.82 4.05 5.85 50.3 45.2 44.8 51.5 42.1 42.3 62.5 48.7 46.6 V 198±27 210±26 216±27 194 208 232 78.3 85.4 201 92.5 156 191 206 222 220 Cr 215±7 267±20 233±17 113 147 124 75.5 40.3 75.8 86.2 41.0 74.9 231 264 223 Co / / / 5.32 0.44 1.70 5.46 0.65 2.27 5.35 0.68 2.19 6.09 1.08 2.25 Ni / / / 31.1 6.0 22.4 23.4 2.1 19.6 26.3 3.9 21.6 36.3 9.0 23.3 Cu 30.6±3.3 19.5±3.8 24.8±2.4 33.9 19.0 22.8 26.0 9.8 21.6 28.1 16.6 20.0 31.8 17.6 22.7 Zn 24±1.9 7.4±1.2 17.7±3.3 24.5 6.9 16.5 24.9 7.0 18.5 24.5 7.9 17.0 25.9 7.9 19.3 Ga 70±8 82±15 72±11 40.2 35.6 31.2 19.2 21.3 30.5 22.2 20.1 30.5 72.3 87.0 69.9 Rb / / / 7.27 1.70 6.27 7.31 1.64 6.24 7.26 1.60 6.14 7.94 1.86 6.02 Sr 691±44 345±12 292±17 177 80 71 712 337 304 704 327 301 714 349 279 Y / / / 20.5 15.0 14.2 85.5 62.2 61.4 88.5 61.7 63.3 90.0 56.4 60.4 Zr 886±95 1160±110 986±84 838 1189 995 648 870 893 692 1056 927 914 1303 993 Nb / / / 64.1 39.6 46.5 16.9 4.3 75.7 83.7 91.3 80.6 88.2 96.7 80.4 Cs / / / 0.37 0.08 0.48 0.31 0.07 0.43 0.33 0.06 0.39 0.38 0.08 0.48 Ba / / / 16.9 7.2 10.8 65.4 35.1 47.6 71.2 35.9 46.0 77.5 43.0 51.6 La / / / 93.1 75.5 72.2 259 129 149 260 130 147 259 120 151 Ce / / / 223 111 116 484 253 299 523 255 296 518 247 294 Pr / / / 18.2 11.8 12.5 58.4 21.8 28.6 62.6 22.5 28.8 64.6 22.7 30.5 Nd / / / 63.7 34.3 39.3 218 66 95 233 69.7 95.7 249 76.6 111 Sm / / / 9.94 4.92 5.89 37.3 11.2 15.8 39.5 11.6 16.2 40.1 11.3 16.7 Eu / / / 1.62 0.84 0.98 6.34 2.00 2.86 6.59 2.14 2.94 7.75 2.05 2.96 Gd / / / 6.18 3.11 3.49 22.2 7.93 10.4 23.8 7.77 10.2 29.3 7.77 10.8 Tb / / / 0.84 0.54 0.54 3.19 1.58 1.79 3.35 1.64 1.81 4.11 1.64 1.79 Dy / / / 4.66 3.47 3.15 18.5 11.0 11.8 19.6 11.6 12.1 23.7 11.8 12.6 Ho / / / 0.89 0.68 0.59 3.52 2.35 2.42 3.78 2.49 2.47 4.07 2.33 2.46 Er / / / 2.46 1.85 1.68 9.71 7.15 7.16 10.6 7.45 7.52 12.3 7.45 7.54 Tm / / / 0.37 0.28 0.26 1.51 1.13 1.14 1.61 1.22 1.21 1.88 1.21 1.23 Yb / / / 2.64 1.75 1.65 10.7 8.12 8.10 11.4 8.59 8.41 13.1 8.61 8.45 Lu / / / 0.38 0.25 0.25 1.54 1.23 1.21 1.66 1.28 1.30 1.84 1.26 1.31 Hf / / / 25.3 35.7 29.9 20.4 28.1 28.3 22.9 36.1 30.9 29.5 42.9 32.6 Ta / / / 2.22 0.67 2.01 0.79 0.33 3.30 6.99 6.88 7.19 8.18 9.78 7.68 Tl / / / 0.06 0.01 0.03 0.06 0.01 0.04 0.06 0.01 0.04 0.07 0.01 0.03 Pb 116±9 26.7±5.7 35.7±6.9 75.7 10.4 16.1 94.2 21.3 25.5 99.7 22.2 26.3 115 26.0 32.1 Th / / / 64.9 66.4 48.8 92.4 99.2 85.5 99.9 101 90.0 109 99.5 88.9 U / / / 37.5 35.8 30.0 32.9 32.4 27.9 35.4 34.3 29.6 37.0 34.3 26.9 注:“/”表示该标准物质没有提供此元素的定值。 表 2 氟化氢铵分解和传统硝酸-氢氟酸密闭消解法的过程空白和检出限
Table 2. Process blanks and detection limits for NH4HF2 decomposition and conventional HF-HNO3 closed digestion
待测
元素分析质量
(丰度,%)过程空白(ng/g, n=12) 检出限(μg/g) 1mL氢氟酸
1mL硝酸①分析纯
氟化氢铵优级纯
氟化氢铵1mL氢氟酸-
1mL硝酸①分析纯
氟化氢铵优级纯
氟化氢铵Li 7(92.50) 0.017 0.070 0.034 0.031 0.078 0.062 Be 9(100.00) 0.000 0.002 0.003 0.004 0.008 0.010 Sc 45(100.00) 0.029 0.299 0.049 0.030 0.152 0.066 V 51(99.75) 0.229 0.018 0.019 0.231 0.016 0.013 Cr 52(83.79) 0.230 4.62 0.906 0.144 2.00 0.426 Co 59(100.00) 0.078 0.316 0.010 0.018 0.133 0.007 Ni 60(26.10) 0.036 11.73 0.222 0.072 4.87 0.079 Cu 63(69.17) 0.033 0.152 1.48 0.043 0.038 0.234 Zn 66(27.90) 0.267 0.601 0.800 0.481 0.494 0.322 Ga 71(39.90) 0.003 0.002 0.000 0.008 0.017 0.009 Rb 85(72.17) 0.007 0.028 0.022 0.013 0.019 0.023 Sr 88(82.58) 0.026 0.010 0.011 0.033 0.098 0.013 Y 89(100.00) 0.001 0.010 0.007 0.004 0.029 0.015 Zr 90(51.45) 0.088 0.074 0.069 0.100 0.234 0.072 Nb 93(100.00) 0.001 0.097 0.051 0.004 0.082 0.052 Cs 133(100.00) 0.002 0.002 0.003 0.004 0.007 0.008 Ba 138(71.70) 0.023 0.317 0.193 0.059 0.488 0.390 La 139(99.91) 0.004 0.015 0.014 0.013 0.022 0.029 Ce 140(88.48) 0.007 0.026 0.016 0.023 0.048 0.015 Pr 141(100.00) 0.001 0.003 0.003 0.005 0.006 0.005 Nd 142(27.12) 0.004 0.012 0.007 0.026 0.020 0.009 Sm 152(26.70) 0.002 0.003 0.003 0.010 0.005 0.009 Eu 153(52.20) 0.001 0.001 0.001 0.003 0.001 0.008 Gd 158(24.84) 0.001 0.003 0.003 0.006 0.007 0.012 Tb 159(100.00) 0.000 0.000 0.001 0.000 0.001 0.004 Dy 164(28.20) 0.001 0.002 0.002 0.006 0.009 0.005 Ho 165(100.00) 0.000 0.000 0.001 0.001 0.001 0.003 Er 166(33.60) 0.001 0.001 0.001 0.006 0.005 0.004 Tm 169(100.00) 0.000 0.000 0.000 0.001 0.001 0.002 Yb 174(31.80) 0.001 0.001 0.001 0.008 0.006 0.003 Lu 175(97.41) 0.000 0.000 0.000 0.001 0.001 0.002 Hf 180(35.10) 0.003 0.003 0.004 0.007 0.013 0.010 Ta 181(99.99) 0.010 0.007 0.010 0.033 0.014 0.028 Tl 205(70.48) 0.001 0.001 0.002 0.006 0.002 0.005 Pb 208(52.40) 0.016 0.311 0.151 0.013 0.463 0.374 Th 232(100.00) 0.001 0.010 0.005 0.004 0.041 0.009 U 238(99.27) 0.000 0.002 0.002 0.001 0.010 0.003 注:①数据来源于文献[39]报道的传统硝酸-氢氟酸密闭消解法过程空白和检出限。 表 3 氟化氢铵分解方法的精密度
Table 3. Method precision test for NH4HF2 decomposition
待测元素 测定值
(μg/g)标准偏差
(μg/g)RSD
(%)Li 86.5 3.34 3.86 Be 8.81 0.12 1.39 Sc 62.5 1.57 3.31 V 221 4.25 1.92 Cr 243 7.54 3.10 Co 6.00 0.19 3.21 Ni 36.2 0.76 2.10 Cu 31.4 0.81 2.58 Zn 26.8 2.53 3.30 Ga 85.3 2.45 2.88 Rb 7.94 0.52 8.61 Sr 693 31.9 4.60 Y 90.4 2.91 3.61 Zr 914 24.0 2.35 Nb 95.2 2.41 2.10 Cs 0.36 0.03 7.22 Ba 74.6 2.50 3.35 La 253 5.39 2.13 Ce 513 5.84 1.14 Pr 61.2 1.33 2.18 Nd 234 4.66 1.99 Sm 37.4 1.17 3.14 Eu 7.48 0.58 8.90 Gd 29.2 1.85 5.56 Tb 4.21 0.28 6.76 Dy 23.7 1.60 8.10 Ho 4.10 0.29 7.05 Er 12.2 0.92 7.54 Tm 1.62 0.12 7.43 Yb 13.3 0.56 4.97 Lu 1.83 0.12 6.70 Hf 28.3 0.85 3.51 Ta 7.96 0.26 3.58 Tl 0.064 0.004 6.32 Pb 118 3.25 2.74 Th 103 3.40 3.31 U 37.4 1.62 4.34 -
[1] 龙克树, 付勇, 龙珍, 等.全球铝土矿中稀土和钪的资源潜力分析[J].地质学报, 2019, 93(6):1279-1295. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dizhixb201906009
Long K S, Fu Y, Long Z, et al.Resource potential analysis of REE and Sc in global bauxite[J].Acta Geologica Sinica, 2019, 93(6):1279-1295. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dizhixb201906009
[2] 卢业友, 杨芬.电感耦合等离子体原子发射光谱法测定铝土矿中锂和镓[J].冶金分析, 2017, 37(3):70-73. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201703012
Lu Y Y, Yang F.Determination of lithium and gallium in bauxite by inductively coupled plasma atomic emission spectrometry[J].Metallurgical Analysis, 2017, 37(3):70-73. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201703012
[3] 钟海仁, 孙艳, 杨岳清, 等.铝土矿(岩)型锂资源及其开发利用潜力[J].矿床地质, 2019, 38(4):898-916. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kcdz201904014
Zhong H R, Sun Y, Yang Y Q, et al.Bauxite (aluminum)-type lithium resources and analysis of its development and utilization potential[J].Mineral Deposits, 2019, 38(4):898-916. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kcdz201904014
[4] 严爽, 黄康俊, 付勇, 等.铝土矿中锂同位素分离提纯方法的建立[J].岩矿测试, 2020, 39(1):41-52. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2019081201275
Yan S, Huang K J, Fu Y, et al.The establishment of methods for separating and purifying lithium isotopes in bauxite[J].Rock and Mineral Analysis, 2020, 39(1):41-52. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2019081201275
[5] 金中国, 周家喜, 黄智龙, 等.黔北务-正-道地区典型铝土矿床伴生有益元素锂、镓和钪分布规律[J].中国地质, 2015, 42(6):1910-1918. http://www.cqvip.com/QK/90050X/201506/667212800.html
Jin Z G, Zhou J X, Huang Z L, et al.The distribution of associated elements Li, Sc and Ga in the typical bauxite deposits over the Wuchuan-Zheng'an-Daozhen bauxite ore district, northern Guizhou Province[J].Geology in China, 2015, 42(6):1910-1918. http://www.cqvip.com/QK/90050X/201506/667212800.html
[6] Lu F H, Xiao T F, Lin J, et al.Resources and extraction of Gallium:A review[J].Hydrometallurgy, 2017, 174:105-115. doi: 10.1016/j.hydromet.2017.10.010
[7] 王登红, 王瑞江, 李建康, 等.中国三稀矿产资源战略调查研究进展综述[J].中国地质, 2013, 40(2):361-370. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdizhi201302001
Wang D H, Wang R J, Li J K, et al.The progress in the strategic research survey of rare earth, rare metal and rare-scattered elements mineral resources[J].Geology in China, 2013, 40(2):361-370. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdizhi201302001
[8] Putzolu F, Papa A P, Mondillo N, et al.Geochemical characterization of bauxite deposits from the Abruzzi mining district (Italy)[J].Minerals, 2018, 8(7):298. doi: 10.3390/min8070298
[9] Khosravi M, Abedini A, Alipour S, et al.The Darzi-Vali bauxite deposit, West-Azarbaidjan Province, Iran:Critical metals distribution and parental affinities[J].Journal of African Earth Sciences, 2017, 129:960-972. doi: 10.1016/j.jafrearsci.2017.02.024
[10] Torró L, Proenza J A, Aiglsperger T, et al.Geological, geochemical and mineralogical characteristics of REE-bearing Las Mercedes bauxite deposit, Dominican Republic[J].Ore Geology Reviews, 2017, 89:114-131. doi: 10.1016/j.oregeorev.2017.06.017
[11] 《岩石矿物分析》编委会.岩石矿物分析(第四版第三分册)[M].北京:地质出版社, 2011:255-256.
The editorial committee of Rock and Mineral Analysis.Rock and mineral analysis (The fourth edition:Volume Ⅲ)[M].Beijing:Geological Publishing House, 2011:255-256.
[12] 胡宝珍.罗丹明B萃取光度法测定铝土矿中镓的质量保证[J].冶金分析, 2005, 25(2):95-96. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx200502028
Hu B Z.Quality assurance of determination of gallium in bauxite by Rhodamine B extraction photometry[J].Metallurgical Analysis, 2005, 25(2):95-96. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx200502028
[13] 朱鲜红, 李德生, 张晶华, 等.乙酸丁酯萃取火焰原子吸收光谱法测定铝土矿中微量镓[J].冶金分析, 2004, 24(6):63-65. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx200406019
Zhu X H, Li D S, Zhang J H, et al.Determination of micro gallium in bauxite by butyl acetate extraction and flame atomic absorption spectrometry[J].Metallurgical Analysis, 2004, 24(6):63-65. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx200406019
[14] Calagari A A, Abedini A.Geochemical investigations on Permo-Triassic bauxite horizon at Kanisheeteh, east of Bukan, west Azarbaidjan, Iran[J].Journal of Geochemical Exploration, 2007, 94:1-18. doi: 10.1016/j.gexplo.2007.04.003
[15] Peh Z, Galović E K.Geochemistry of Istrian Lower Palae-ogene bauxites-Is it relevant to the extent of subaerial exposure during Cretaceous times?[J].Ore Geology Reviews, 2014, 63:296-306. doi: 10.1016/j.oregeorev.2014.05.020
[16] Cotta A J B, Enzweiler J.Classical and new procedures of whole rock dissolution for trace element determination by ICP-MS[J].Geostandards and Geoanalytical Research, 2011, 36(1):27-50. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1bf9b3e287948e3c1f181d920b8981c7
[17] Zhang W, Hu Z C, Liu Y S, et al.Reassessment of HF/HNO3 decomposition capability in the high-pressure digestion of felsic rocks for multi-element determination by ICP-MS[J].Geostandards and Geoanalytical Research, 2012, 36(3):271-278. doi: 10.1111/j.1751-908X.2012.0156.x
[18] 王琰, 孙洛新, 张帆, 等.电感耦合等离子体发射光谱法测定含刚玉的铝土矿中硅铝铁钛[J].岩矿测试, 2013, 32(5):719-723. http://www.ykcs.ac.cn/article/id/b77e549e-dbd2-49c3-96ce-9d0075b681e6
Wang Y, Sun L X, Zhang F, et al.Determination of Si, Al, Fe and Ti in bauxite by inductively coupled plasma-atomic emission spectrometry[J].Rock and Mineral Analysis, 2013, 32(5):719-723. http://www.ykcs.ac.cn/article/id/b77e549e-dbd2-49c3-96ce-9d0075b681e6
[19] 孙红宾, 刘贵磊, 赵怀颖, 等.偏硼酸锂熔融-ICP-AES法测定含刚玉铝土矿中主成分[J].分析试验室, 2017, 36(12):1429-1434. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxsys201712013
Sun H B, Liu G L, Zhao H Y, et al.Determination of main components in corundum-bearing bauxite by ICP-AES with lithium metaborate fusion method[J].Chinese Journal of Analysis Laboratory, 2017, 36(12):1429-1434. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxsys201712013
[20] 杨小丽, 李小丹, 邹棣华.溶样方法对电感耦合等离子体质谱法测定铝土矿中稀土元素的影响[J].冶金分析, 2016, 36(7):56-62. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201607009
Yang X L, Li X D, Zou D H.Influence of sample dissolution method on determination of rare earth elements in bauxite by inductively coupled plasma mass spectrometry[J]. Metellurgical Analysis, 2016, 36(7):56-62. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201607009
[21] da Costa M L, da Silva Cruz G, de Almeida H D F, et al.On the geology, mineralogy and geochemistry of the bauxite-bearing regolith in the Lower Amazon Basin:Evidence of genetic relationship[J].Journal of Geochemical Exploration, 2014, 146:58-74. doi: 10.1016/j.gexplo.2014.07.021
[22] Monsels D A, van Bergen M J.Bauxite formation on Proterozoic bedrock of Suriname[J].Journal of Geochemical Exploration, 2017, 180:71-90. doi: 10.1016/j.gexplo.2017.06.011
[23] Mongelli G, Boni M, Buccione R, et al.Geochemistry of the Apulian karst bauxites (southern Italy):Chemical fractionation and parental affinities[J].Ore Geology Reviews, 2014, 63:9-21. doi: 10.1016/j.oregeorev.2014.04.012
[24] Ahmadnejad F, Zamanian H, Taghipour B, et al.Mineralogical and geochemical evolution of the Bidgol bauxite deposit, Zagros Mountain Belt, Iran:Implications for ore genesis, rare earth elements fractionation and parental affinity[J].Ore Geology Reviews, 2017, 86:755-783. doi: 10.1016/j.oregeorev.2017.04.006
[25] 杨载明.电感耦合等离子体发射光谱法测定铝土矿样品中镓三种前处理方法的比较[J].岩矿测试, 2011, 30(3):315-317. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ykcs201103014
Yang Z M.The comparison of three sample pretreatment methods in determination of gallium in bauxite ores by inductively coupled plasma-atomic emission spectro-metry[J].Rock and Mineral Analysis, 2011, 30(3):315-317. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ykcs201103014
[26] 高志军, 陈静, 陈浩凤, 等.熔融制样-X射线荧光光谱法测定硅酸盐和铝土矿中主次组分[J].冶金分析, 2015, 35(7):73-78. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201507013
Gao Z J, Chen J, Chen H F, et al.Simultaneous determination of major and minor components in silicate and bauxite by X-ray fluorescence[J].Metellurgical Analysis, 2015, 35(7):73-78. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yjfx201507013
[27] 胡璇, 石磊, 张炜华.碱熔融-电感耦合等离子体发射光谱法测定高硫铝土矿中的硫[J].岩矿测试, 2017, 36(2):124-129. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2017.02.005
Hu X, Shi L, Zhang W H.Determination of sulfur in high-sulfur bauxite by alkali fusion-inductively coupled plasma-optical emission spectrometry[J].Rock and Mineral Analysis, 2017, 36(2):124-129. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2017.02.005
[28] Awaji S, Nakamura K, Nozaki T, et al.A simple method for precise determination of 23 trace elements in granitic rocks by ICP-MS after lithium tetraborate fusion[J].Resource Geology, 2006, 56(4):471-478. doi: 10.1111/j.1751-3928.2006.tb00299.x
[29] Zhang W, Hu Z C, Liu Y S, et al.Quantitative analysis of major and trace elements in NH4HF2-modified silicate rock powders by laser ablation-inductively coupled plasma mass spectrometry[J].Analytica Chimica Acta, 2017, 983(29):149-159. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f4687d8b182cc968dd8d014647a13f25
[30] Zhang W, Hu Z C.Recent advances in sample prepa-ration methods for elemental and isotopic analysis of geological samples[J].Spectrochimica Acta Part B, 2019, 160:105690. doi: 10.1016/j.sab.2019.105690
[31] Magaldi T T, Navarro M S, Enzweiler J.Assessment of dissolution of silicate rock reference materials with ammonium bifluoride and nitric acid in a microwave oven[J].Geostandards and Geoanalytical Research, 2019, 43(1):189-208. doi: 10.1111/ggr.12242
[32] Ayranci B.A rapid decomposition method for analyzing zirconia[J].Mineral Research and Exploration Bulletin, 1989, 109:75-79. http://www.researchgate.net/publication/237446337_A_rapid_decomposition_method_for_analyzing_zirconia
[33] Mariet C, Belhadj O, Leroy S, et al.Relevance of NH4F in acid digestion before ICP-MS analysis[J].Talanta, 2008, 77(2):445-450. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e2c02d5be104173a0d6febd89f2139bd
[34] Hu Z C, Gao S, Liu Y S, et al.NH4F assisted high pressure digestion of geological samples for multi-element analysis by ICP-MS[J].Journal of Analytical Atomic Spectrometry, 2010, 25(3):408-413. doi: 10.1039/b921006g
[35] Zhang W, Hu Z C, Liu Y S, et al.Total rock dissolution using ammonium bifluoride (NH4HF2) in screw-top teflon vials:A new development in open-vessel digestion[J].Analytical Chemistry, 2012, 84(23):10686-10693. http://dx.doi.org/10.1021/ac302327g
[36] Hu Z C, Zhang W, Liu Y S, et al.Rapid bulk rock decomposition by ammonium fluoride (NH4F) in open vessels at an elevated digestion temperature[J].Chemical Geology, 2013, 355:144-152. doi: 10.1016/j.chemgeo.2013.06.024
[37] Zhang W, Qi L, Hu Z C, et al.An investigation of diges-tion methods for trace elements in bauxite and their determination in ten bauxite reference materials using inductively coupled plasma-mass spectrometry[J].Geostandards and Geoanalytical Research, 2016, 40(2):195-216. doi: 10.1111/j.1751-908X.2015.00356.x
[38] Yokoyama T, Makishima A, Nakamura E.Evaluation of the coprecipitation of incompatible trace elements with fluoride during silicate rock dissolution by acid digestion[J].Chemical Geology, 1999, 157:175-187. doi: 10.1016/S0009-2541(98)00206-X
[39] Hu Z C, Gao S.Upper crustal abundances of trace elements:A revision and update[J].Chemical Geology, 2008, 253(3-4):205-221. doi: 10.1016/j.chemgeo.2008.05.010
-




 下载:
下载: