Characterization of Binary Hydrates Containing Methane by X-ray Diffraction and Microscopic Laser Raman Spectroscopy
-
摘要:
天然气水合物的晶体结构主要取决于客体分子种类与组成,目前单组分水合物的结构和谱学特征较为明确,但多组分水合物相关研究较少。为解决多组分水合物的结构识别问题,探讨其谱学特征,本文实验合成了甲烷-丙烷(CH4-C3H8)和甲烷-四氢呋喃(CH4-THF)两种含CH4双组分水合物以及CH4、C3H8和THF等三种单组分水合物,并采用低温X射线粉晶衍射(PXRD)和显微激光拉曼光谱进行了表征。结果表明:CH4-C3H8和CH4-THF双组分水合物的晶格常数a分别为17.2312×10-10m和17.2241×10-10m,为典型的Ⅱ型结构水合物,与相应C3H8和THF单组分水合物结构相同。在CH4-C3H8水合物中,CH4在大、小笼中均有分布,呈现两个特征拉曼峰(2900cm-1和2911cm-1);C3H8仅分布在大笼,与单组分水合物相比,其C—H伸缩振动峰峰位几无变化,而C—C伸缩振动峰(873cm-1)向低频迁移约3cm-1。在CH4-THF水合物中,大笼被THF占据,CH4仅填充在小笼中(2910cm-1);双组分水合物中,THF分子C—C和C—H伸缩振动峰峰位均与单组分水合物基本一致。分析认为,含CH4双组分水合物的结构类型与其相应的大分子水合物一致,大分子对双组分水合物的晶体结构特征具有决定作用。同时,大分子影响了CH4分子在笼型结构中的分布,致使双组分水合物的拉曼光谱特征存在显著差异。研究结论对基于谱学特征识别多组分水合物微观结构具有重要的指导意义。
Abstract:BACKGROUND The crystal structure of natural gas hydrate mainly depends on the species and composition of guest molecules. At present, the structure and spectral characteristics of single-component hydrate are relatively clear, but the studies of multi-component hydrates are relatively scarce.
OBJECTIVES To solve the problem of structure identification of multi-component hydrate, and understand its spectral characteristics.
METHODS Methane-propane (CH4-C3H8) and methane-tetrahydrofuran (CH4-THF) binary hydrates, as well as CH4, C3H8 and THF (molar ratio 1:17) single-component hydrate samples were synthesized and characterized by low-temperature X-ray powder diffraction (PXRD) and Raman spectroscopy.
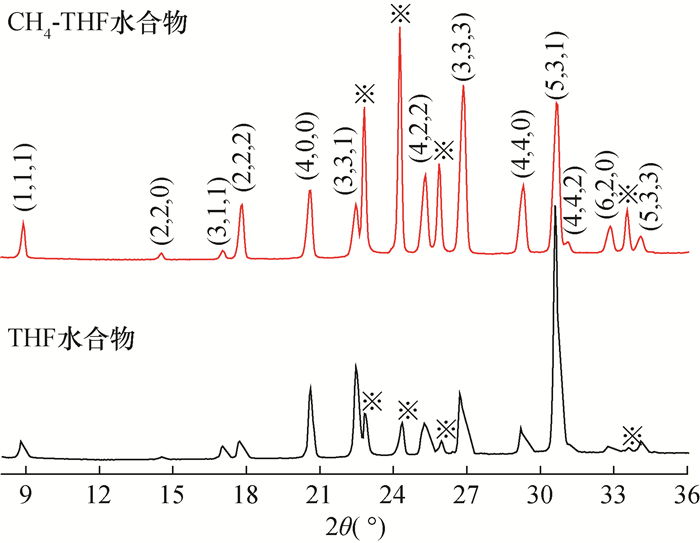
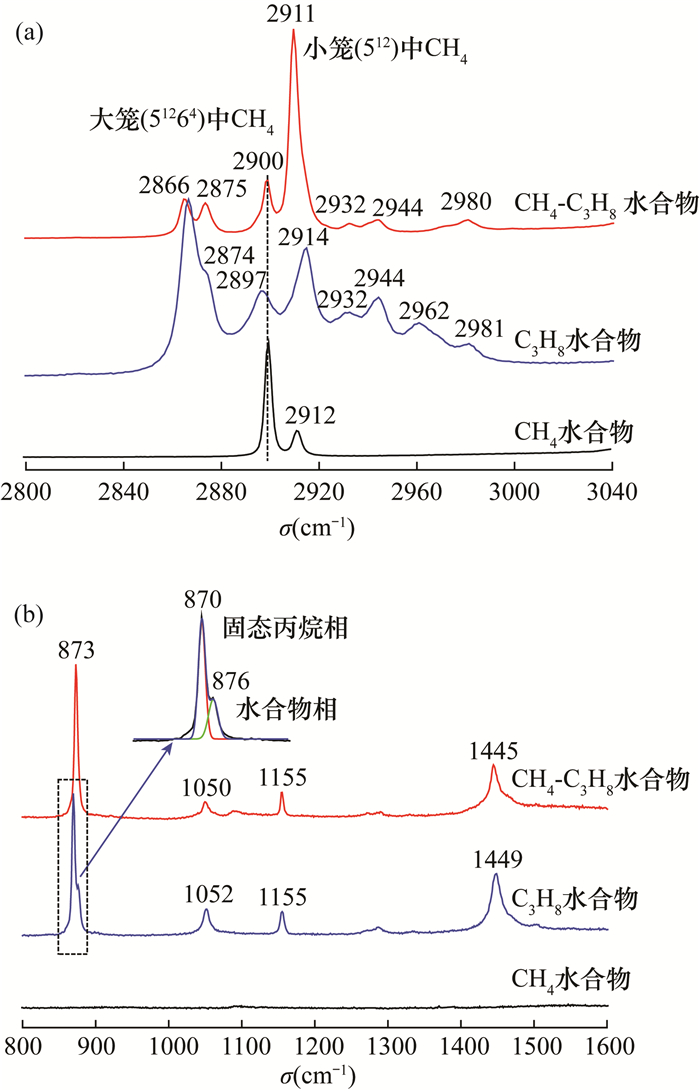
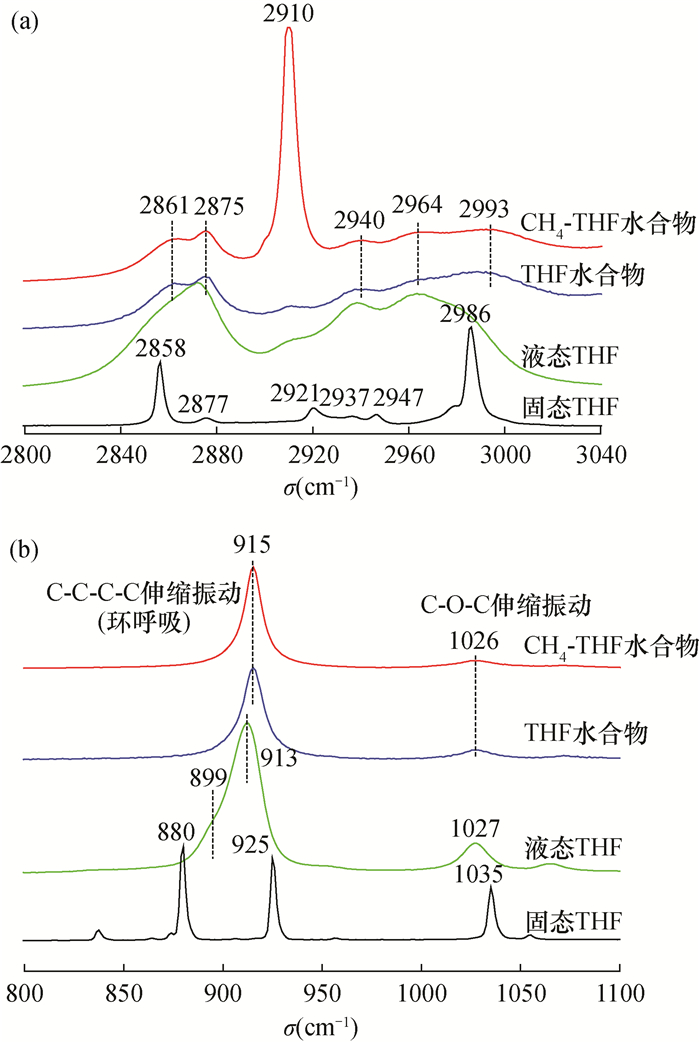
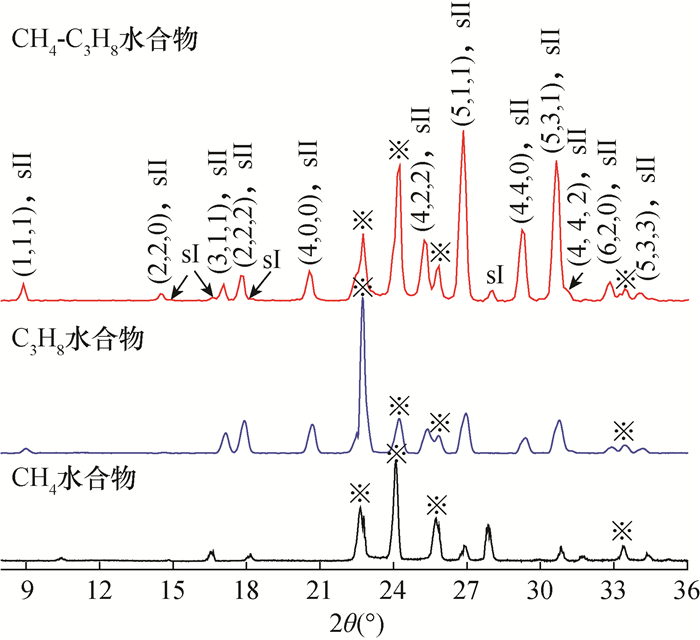
RESULTS Both of the binary hydrates were typical structure Ⅱ hydrates, which were the same as those of C3H8 and THF hydrate. The crystal cell parameters a for CH4-C3H8 and CH4-THF binary hydrates were 17.2312×10-10 m and 17.2241×10-10 m, respectively. For CH4-C3H8 hydrate, CH4 was distributed in both large and small cages, showing two characteristic Raman peaks (2900cm-1 and 2911cm-1); C3H8 was only distributed in the large cage. Compared with C3H8 hydrate, the C-H stretching vibration peak was almost unchanged while the C-C stretching vibration peak (873cm-1) shifted by 3cm-1 to low frequency. For CH4-THF hydrate, the large cage was occupied by THF and CH4 was only filled in the small cage (2910cm-1). The peak positions of C-C and C-H stretching vibrations of THF in CH4-THF hydrate were consistent with those of THF hydrate.
CONCLUSIONS The structure types of binary hydrates are consistent with those of single component hydrates. Molecules with larger sizes play a key role in the crystal structure of binary hydrates containing CH4. Moreover, the distribution of CH4 molecules in the cages are also influenced by larger molecules, and the Raman spectral characteristics of binary hydrates are significantly different. The conclusion of this study has important guiding significance for identifying the microstructure of multi-component hydrate based on spectral characteristics.
-

-
表 1 水合物样品生成前后压力变化
Table 1. Pressure change before and after hydrate formation
水合物样品 初始压力(MPa) 终止压力(MPa) CH4 8.0 6.6 C3H8 0.5 0.3 THF(5.56mol·%) 常压 常压 CH4-C3H8 7.0 4.9 CH4-THF 8.3 5.5 表 2 CH4-C3H8水合物样品客体分子拉曼峰及其归属
Table 2. Raman peaks and assignment of guest molecules in CH4-C3H8 hydrate samples
峰位(cm-1) 客体分子 振动模式 笼型分布 2980 C3H8 C—H伸缩 Ⅱ,51264 2932 C3H8 C—H伸缩 Ⅱ,51264 2911 CH4 C—H伸缩 Ⅱ,512 2900 CH4 C—H伸缩 Ⅱ,51264 2875 C3H8 C—H伸缩 Ⅱ,51264 2866 C3H8 C—H伸缩 Ⅱ,51264 1449 CH4,C3H8 CH2剪式 Ⅱ,51264 1445 CH4,C3H8 CH2剪式 Ⅱ,51264 1155 C3H8 (CH3)2CH伸缩 Ⅱ,51264 1050 C3H8 C—C骨架 Ⅱ,51264 873 C3H8 C—C伸缩 Ⅱ,51264 表 3 CH4-THF水合物主要拉曼峰及其归属
Table 3. Raman peaks and assignments of guest molecules in CH4-THF hydrate samples
峰位(cm-1) 客体分子 振动模式 笼型分布 2993 THF C—H伸缩振动 Ⅱ,51264 2964 THF C—H伸缩振动 Ⅱ,51264 2940 THF C—H伸缩振动 Ⅱ,51264 2910 CH4 C—H伸缩振动 Ⅱ,512 2875 THF C—H伸缩振动 Ⅱ,51264 2861 THF C—H伸缩振动 Ⅱ,51264 1026 THF C—O—C伸缩振动 Ⅱ,51264 915 THF C—C—C—C伸缩振动 Ⅱ,51264 表 4 水合物样品晶体结构参数
Table 4. Crystal structure parameters of hydrate samples
水合物样品 晶系 空间群 晶格常数
a=b=c(×10-10m)结构类型 CH4 体心立方 Pm3n 11.8927 Ⅰ C3H8 面心立方 Fd3m 17.2029 Ⅱ THF(摩尔比1∶17) 面心立方 Fd3m 17.2180 Ⅱ CH4-C3H8 面心立方 Fd3m 17.2312 Ⅱ CH4-THF 面心立方 Fd3m 17.2241 Ⅱ -
[1] Li J, Ye J, Qin X, et al. The first offshore natural gas hydrate production test in South China Sea[J]. China Geology, 2018, 1(1): 5-16. doi: 10.31035/cg2018003
[2] 叶建良, 秦绪文, 谢文卫, 等. 中国南海天然气水合物第二次试采主要进展[J]. 中国地质, 2020, 47(3): 557-568. https://www.cnki.com.cn/Article/CJFDTOTAL-DIZI202003002.htm
Ye J L, Qin X W, Xie W W, et al. Main progress of the second gas hydrate trial production in the South China Sea[J]. Geology in China, 2020, 47(3): 557-568. https://www.cnki.com.cn/Article/CJFDTOTAL-DIZI202003002.htm
[3] Sloan E D. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426(6964): 353-359. doi: 10.1038/nature02135
[4] Liu C, Meng Q, He X, et al. Characterization of natural gas hydrate recovered from Pearl River Mouth Basin in South China Sea[J]. Marine and Petroleum Geology, 2015, 61: 14-21. doi: 10.1016/j.marpetgeo.2014.11.006
[5] Liu C, Meng Q, Hu G, et al. Characterization of hydrate-bearing sediments recovered from the Shenhu area of the South China Sea[J]. Interpretation, 2017, 5(3): SM13-SM23. doi: 10.1190/INT-2016-0211.1
[6] 孟庆国, 刘昌岭, 李承峰, 等. 青海聚乎更钻探区天然气水合物拉曼光谱特征[J]. 现代地质, 2015, 29(5): 200-208. https://www.cnki.com.cn/Article/CJFDTOTAL-XDDZ201505023.htm
Meng Q G, Liu C L, Li C F, et al. Raman spectroscopic characteristics of natural gas hydrates from Juhugeng drilling area, Qinghai[J]. Geoscience, 2015, 29(5): 200-208. https://www.cnki.com.cn/Article/CJFDTOTAL-XDDZ201505023.htm
[7] Wei J, Fang Y, Lu H, et al. Distribution and characteristics of natural gas hydrates in the Shenhu Sea Area, South China Sea[J]. Marine and Petroleum Geology, 2018, 98: 622-628. doi: 10.1016/j.marpetgeo.2018.07.028
[8] Yu Y S, Zhang Q Z, Li X S, et al. Kinetics, compositions and structures of carbon dioxide/hydrogen hydrate formation in the presence of cyclopentane[J]. Applied Energy, 2020, 265: 114808. doi: 10.1016/j.apenergy.2020.114808
[9] 夏宁, 刘昌岭, 业渝光, 等. 显微激光拉曼光谱测定天然气水合物的方法研究[J]. 岩矿测试, 2011, 30(4): 416-422. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201809090102
Xia N, Liu C L, Ye Y G, et al. Study on determination method of natural gas hydrates by micro-laser Raman spectroscopy[J]. Rock and Mineral Analysis, 2011, 30(4): 416-422. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201809090102
[10] Cho S J, Hai T L S, Lee J D. In-situ Raman and kinetic study on the methane hydrate formation and decomposition[J]. Energy Procedia, 2019, 158: 5615-5621. doi: 10.1016/j.egypro.2019.01.578
[11] 孟庆国, 刘昌岭, 业渝光, 等. 甲烷水合物分解过程原位激光拉曼光谱观测[J]. 天然气工业, 2010, 30(6): 117-120. https://www.cnki.com.cn/Article/CJFDTOTAL-TRQG201006038.htm
Meng Q G, Liu C L, Ye Y G, et al. In situ Raman spectroscopic observation on methane hydrate dissociation[J]. Natural Gas Industry, 2010, 30(6): 117-120. https://www.cnki.com.cn/Article/CJFDTOTAL-TRQG201006038.htm
[12] 张保勇, 周泓吉, 吴强, 等. 不同驱动力下瓦斯水合物生长过程Raman光谱特征[J]. 光谱学与光谱分析, 2017, 37(9): 118-123. https://www.cnki.com.cn/Article/CJFDTOTAL-GUAN201709023.htm
Zhang B Y, Zhou H J, Wu Q, et al. Raman spectra characteristics of gas hydrate growth with different driving forces[J]. Spectroscopy and Spectral Analysis, 2017, 37(9): 118-123. https://www.cnki.com.cn/Article/CJFDTOTAL-GUAN201709023.htm
[13] Guo D, Ou W, Ning F, et al. The effects of hydrate formation and dissociation on the water-oil interface: Insight into the stability of an emulsion[J]. Fuel, 2020, 266: 116980. doi: 10.1016/j.fuel.2019.116980
[14] Li Z, Holzammer C C, Braeuer A S. Analysis of the dis-solution of CH4/CO2 mixtures into liquid water and the subsequent hydrate formation via in situ Raman spectroscopy[J]. Energies, 2020, 13(4): 793. doi: 10.3390/en13040793
[15] Lee Y, Choi W, Seo Y J, et al. Structural transition induced by cage-dependent guest exchange in CH4+C3H8 hydrates with CO2 injection for energy recovery and CO2 sequestration[J]. Applied Energy, 2018, 228: 229-239. doi: 10.1016/j.apenergy.2018.06.088
[16] Tang C, Zhou X, Li D, et al. In situ Raman investigation on mixed CH4-C3H8 hydrate dissociation in the presence of polyvinylpyrrolidone[J]. Fuel, 2018, 214: 505-511. doi: 10.1016/j.fuel.2017.11.063
[17] Fang B, Ning F, Cao P, et al. Modeling thermodynamic properties of propane or tetrahydrofuran mixed with carbon dioxide or methane in structure-Ⅱ clathrate hydrates[J]. The Journal of Physical Chemistry C, 2017, 121(43): 23911-23925. doi: 10.1021/acs.jpcc.7b06623
[18] Shi L, Ding J, Liang D. Enhanced CH4 storage in hydrates with the presence of sucrose stearate[J]. Energy, 2019, 180: 978-988. doi: 10.1016/j.energy.2019.05.151
[19] Kumar A, Veluswamy H P, Kumar R, et al. Direct use of seawater for rapid methane storage via clathrate (sⅡ) hydrates[J]. Applied Energy, 2019, 235: 21-30. doi: 10.1016/j.apenergy.2018.10.085
[20] Khurana M, Veluswamy H P, Daraboina N, et al. Therm odynamic and kinetic modelling of mixed CH4-THF hydrate for methane storage application[J]. Chemical Engineering Journal, 2019, 370: 760-771. doi: 10.1016/j.cej.2019.03.172
[21] Kumar A, Veluswamy H P, Linga P, et al. Molecular level investigations and stability analysis of mixed methane-tetrahydrofuran hydrates: Implications to energy storage[J]. Fuel, 2019, 236: 1505-1511. doi: 10.1016/j.fuel.2018.09.126
[22] Castillo-Borja F, Bravo-Sánchez U I, Vázquez-Román R, et al. Biogas purification via sⅡ hydrates in the presence of THF and DMSO solutions using MD simulations[J]. Journal of Molecular Liquids, 2020, 297: 111904. doi: 10.1016/j.molliq.2019.111904
[23] Dong Q B, Su W, Liu X W, et al. Separation of the N2/CH4 mixture through hydrate formation in ordered mesoporous carbon[J]. Adsorption Science & Technology, 2014, 32(10): 821-832. http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=100517248&site=ehost-live
[24] 孟庆国. 多组分气体水合物结构特征及生成分解过程研究[D]. 北京: 中国地质科学院, 2019.
Meng Q G.Research on the multi-component gas hydrates: Structure characteristics, formation and dissociation process[D].Beijing: Chinese Academy of Geological Sciences, 2019.
[25] 刘昌岭, 孟庆国. X射线衍射法在天然气水合物研究中的应用[J]. 岩矿测试, 2014, 33(4): 468-479. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201809090102
Liu C L, Meng Q G. Applications of X-ray diffraction in natural gas hydrate research[J]. Rock and Mineral Analysis, 2014, 33(4): 468-479. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201809090102
[26] 孟庆国, 刘昌岭, 李承峰, 等. 常见客体分子对笼型水合物晶格常数的影响[J]. 物理化学学报, 2020, 36. doi:10.3866/PKU.WHXB201910010.
Meng Q G, Liu C L, Li C F, et al. Effect of common guest molecules on the lattice constants of clathrate hydrates[J]. Acta Physico-Chimica Sinica, 2020, 36. doi:10.3866/PKU.WHXB201910010.
[27] 田苗, 孟庆国, 刘昌岭, 等. 天然气水合物粉晶X射线衍射测试参数优化及分析方法[J]. 岩矿测试, 2017, 36(5): 481-488. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201809090102
Tian M, Meng Q G, Liu C L, et al. Parameter optimization and analysis method for determination of natural gas hydrate by powder X-ray diffraction[J]. Rock and Mineral Analysis, 2017, 36(5): 481-488. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201809090102
[28] Kim E, Seo Y. A novel discovery of a gaseous sH clath-rate hydrate former[J]. Chemical Engineering Journal, 2019, 359: 775-778. doi: 10.1016/j.cej.2018.11.170
[29] Xu C G, Yan R, Fu J, et al. Insight into micro-mechanism of hydrate-based methane recovery and carbon dioxide capture from methane-carbon dioxide gas mixtures with thermal characterization[J]. Applied Energy, 2019, 239: 57-69. doi: 10.1016/j.apenergy.2019.01.087
[30] Takeya S, Kamata Y, Uchida T, et al. Coexistence of structureⅠand Ⅱ hydrates formed from a mixture of methane and ethane gases[J]. Canadian Journal of Physics, 2003, 81(1-2): 479-484. doi: 10.1139/p03-038
[31] Menezes D E S D, Sum A K, Desmedt A, et al. Coexi-stence of sⅠ and sⅡ in methane-propane hydrate former systems at high pressures[J]. Chemical Engineering Science, 2019, 208: 115149. doi: 10.1016/j.ces.2019.08.007
[32] Yu C, Chen L, Sun B. Experimental characterization of guest molecular occupancy in clathrate hydrate cages: A review[J]. Chinese Journal of Chemical Engineering, 2019, 27: 2189-2206. doi: 10.1016/j.cjche.2019.03.026
[33] Hiraga Y, Sasagawa T, Yamamoto S, et al. A precise deconvolution method to derive methane hydrate cage occupancy ratios using Raman spectroscopy[J]. Chemical Engineering Science, 2019, 214: 115361. http://www.sciencedirect.com/science/article/pii/S0009250919308516
[34] Prasad P S R, Sowjanya Y, Prasad K S. Micro-Raman investigations of mixed gas hydrates[J]. Vibrational Spectroscopy, 2009, 50(2): 319-323. doi: 10.1016/j.vibspec.2009.02.003
[35] 孟庆国, 刘昌岭, 贺行良, 等. 祁连山冻土区天然气水合物激光拉曼光谱特征[J]. 地质通报, 2011, 30(12): 1863-1867. https://www.cnki.com.cn/Article/CJFDTOTAL-ZQYD201112009.htm
Meng Q G, Liu C L, He X L, et al. Laser-Raman spectroscopy characteristics of natural gas hydrates from Qilian Mountain permafrost[J]. Geological Bulletin of China, 2011, 30(12): 1863-1867. https://www.cnki.com.cn/Article/CJFDTOTAL-ZQYD201112009.htm
[36] Prasad P S R, Chari V D. Preservation of methane gas in the form of hydrates: Use of mixed hydrates[J]. Journal of Natural Gas Science & Engineering, 2015, 25: 10-14. http://www.sciencedirect.com/science/article/pii/S1875510015001778
[37] Truong-Lam H S, Seo S D, Kim S, et al. In situ Raman study of the formation and dissociation kinetics of methane and methane/propane hydrates[J]. Energy & Fuels, 2020, 34(5): 6288-6297. http://pubs.acs.org/doi/abs/10.1021/acs.energyfuels.0c00813
[38] 孟庆国, 刘昌岭, 业渝光, 等. 13C固体核磁共振法测定CH4-THF二元水合物的微观结构特征[J]. 天然气工业, 2015, 35(3): 135-140. https://www.cnki.com.cn/Article/CJFDTOTAL-TRQG201503028.htm
Meng Q G, Liu C L, Ye Y G, et al. Measurement of micro-structure features of binary CH4-THF clathrate hydrate based on the 13C solid state NMR[J]. Natural Gas Industry, 2015, 35(3): 135-140. https://www.cnki.com.cn/Article/CJFDTOTAL-TRQG201503028.htm
[39] Tulk C A, Klug D D, Ripmeester J A. Raman spectro-scopic studies of THF clathrate hydrate[J]. Journal of Physical Chemistry A, 1998, 102(45): 8734-8739. doi: 10.1021/jp981497q
-



 下载:
下载: