Distribution and Origin of High Arsenic and Fluoride in Groundwater of the North Henan Plain
-
摘要:
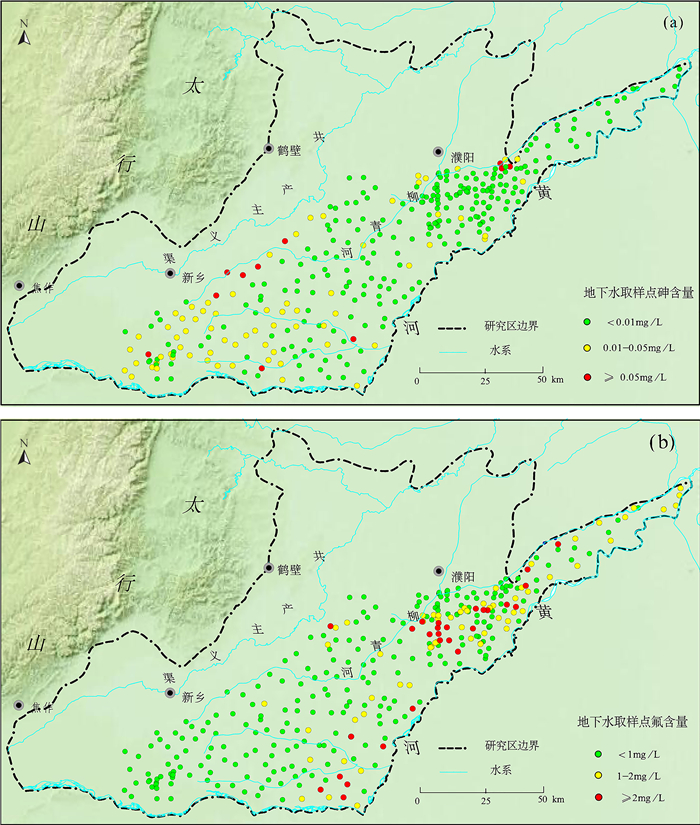
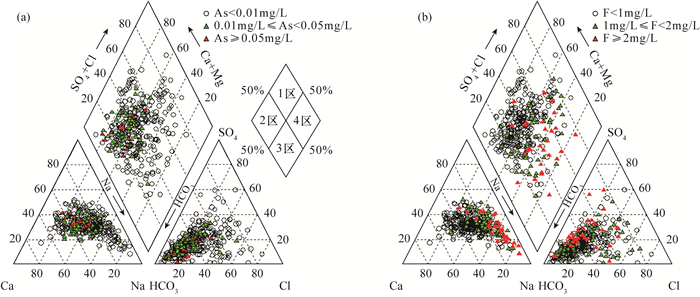
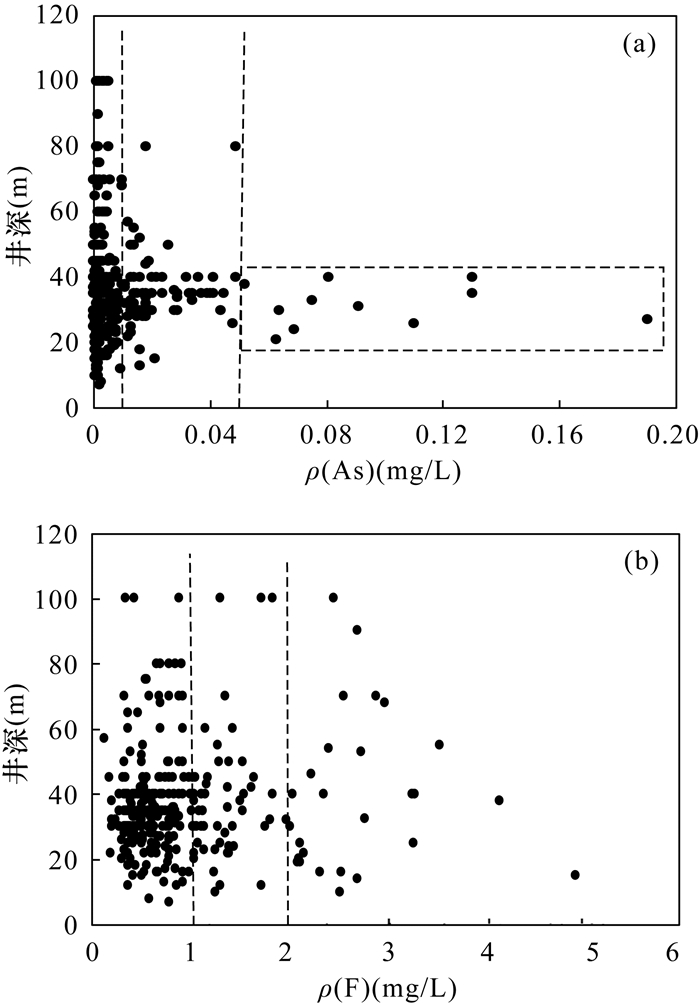
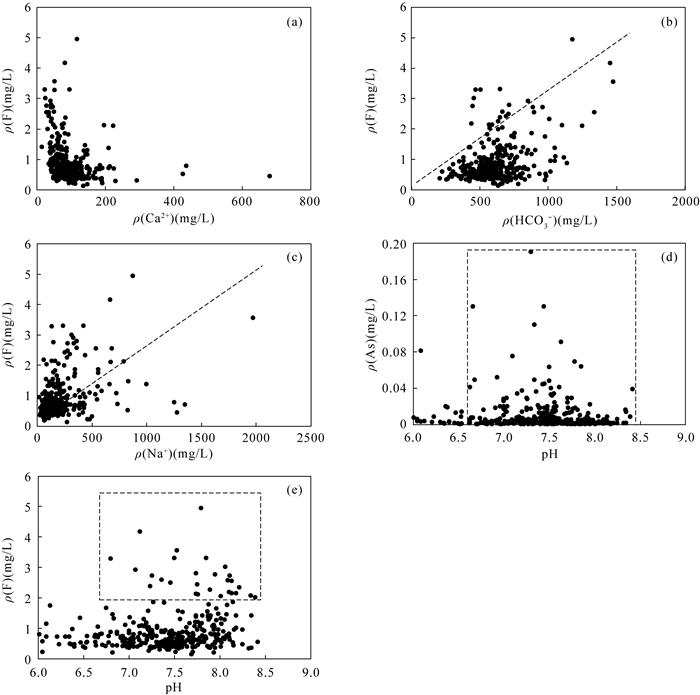
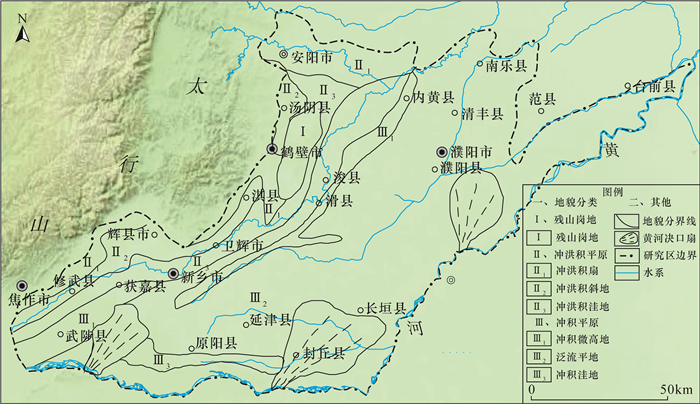
豫北平原地处黄河中下游,同时存在着高砷和高氟地下水,但目前这种零星分布区砷和氟的共存机制尚不明确。本文采集了豫北平原332组浅层地下水样品,采用原子荧光光谱法测定砷含量,离子色谱和电感耦合等离子体发射光谱等方法测定氟及其他阴阳离子含量,探讨地下水中砷和氟的空间分布规律,并结合水化学图解和因子分析法提取出影响该区地下水演化的主要因子,以此为思路对该区高砷和高氟地下水的成因机制进行探讨。结果表明:该平原地下水中砷和氟的浓度范围分别为0.0001~0.1900mg/L和0.13~4.94mg/L。高砷地下水主要分布在太行山前冲洪积洼地和黄河决口扇垂向15~80m;高氟地下水分布于黄河沿岸的黄河现代河道影响带垂向7~100m。蒸发浓缩作用、矿物的溶解/解吸附作用和氧化还原环境是控制该区地下水演化的主要因子。氟在因子F1(蒸发浓缩作用)和F2(矿物的溶解/解吸附作用)中分别占有0.214和0.743的载荷,氟浓度与ρ(Na+)/[ρ(Na+)+ρ(Ca2+)]呈正相关,高浓度氟出现在Ca2+浓度较低的地下水中。黄河现代河道影响带强烈的蒸发浓缩作用有助于含氟矿物的溶解,黄河水的灌溉增加了地下水中Na+浓度,进一步增强了其溶解作用,在这种环境下氟会浓缩并富集在地下水中。砷在因子F3(氧化还原环境)中占有0.728的载荷,与Fe2+、NH4+呈正相关,与NO3-、SO42-呈负相关,Eh越低,砷浓度越高。太行山前冲洪积洼地和黄河决口扇的还原环境有利于含砷的铁氧化物/氢氧化物发生还原性溶解,从而形成高砷地下水。pH值升高引起的以阴离子形式存在的砷酸根/亚砷酸根/氟化物在矿物表面的解吸附作用有利于该区砷和氟在地下水中共存。然而,该区地下水中砷和氟的相关性并不十分显著,这是由于高砷区高浓度的钙离子不利于氟的富集,而高氟区的弱还原条件不利于含砷铁氧化物/氢氧化物的溶解。本文研究结果探讨了豫北平原地下水中砷和氟的共存机制,进一步丰富了高砷高氟地下水共污染的理论体系。
Abstract:BACKGROUND The North Henan Plain is located in the middle and lower reaches of the Yellow River, and both high arsenic and fluoride groundwater exist. However, the coexistence mechanism of arsenic and fluoride in this sporadic distribution area is unclear.
OBJECTIVES To investigate the spatial distribution characteristics and formation mechanism of arsenic and fluoride in shallow groundwater in the North Henan Plain.
METHODS 332 groups of shallow groundwater samples were collected and analyzed in the North Henan Plain. Atomic fluorescence spectrometry was used to determine arsenic content, and ion chromatography and inductively coupled plasma emission spectroscopy were used to determine the content of fluoride and other cation-anions. Based on the spatial distribution of arsenic and fluoride, combined with hydrochemical diagrams and factor analysis, three main factors affecting the evolution of groundwater in this area were extracted, and the formation mechanism of high arsenic and high fluoride groundwater in this area was discussed.
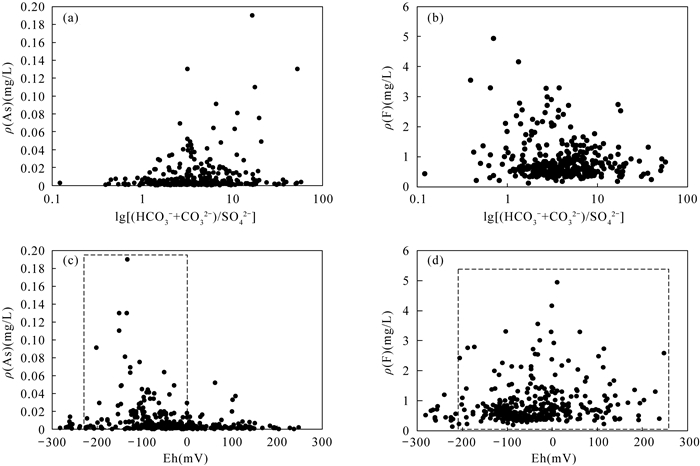
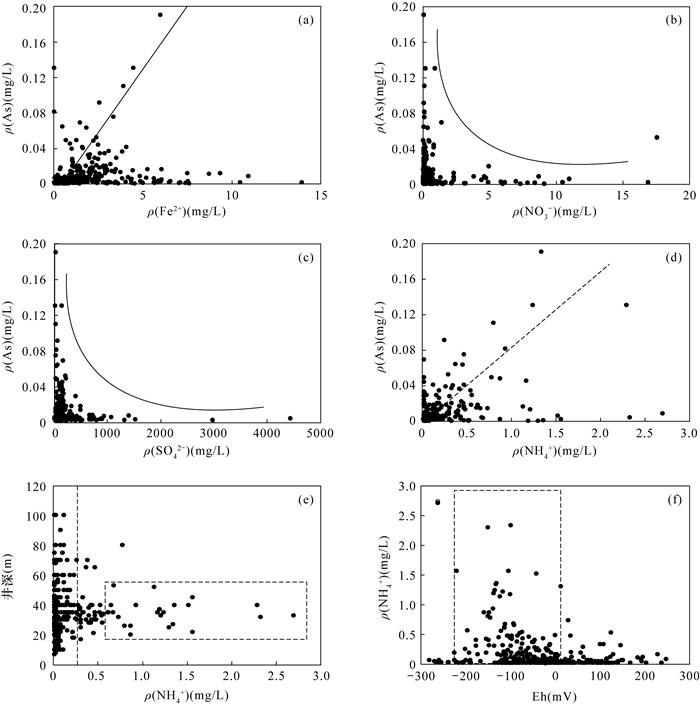
RESULTS The concentrations of arsenic and fluoride in groundwater were 0.0001-0.1900mg/L and 0.13-4.94mg/L, respectively. The high-arsenic groundwater was mainly distributed in the vertical depth of 15-80m in the front alluvial-diluvial depression of Taihang Mountain and the Yellow River flood fan. The high-fluoride groundwater was mainly distributed in the vertical depth of 7-100m in the modern channel influence zone of the Yellow River. Evaporation and concentration, mineral dissolution/desorption, and redox environment were the main factors controlling the evolution of groundwater in this area. Fluoride had loads of 0.214 and 0.743 in factor F1 (evaporation and concentration) and F2 (mineral dissolution/desorption), respectively. High concentration of F appeared in groundwater with low concentration of Ca2+, and the concentration of F was positively correlated with ρ(Na+)/[ρ(Na+)+ρ(Ca2+)]. The strong evaporation and concentration in the modern channel influence zone of the Yellow River contributed to the dissolution of fluorine-containing minerals, and the irrigation of the Yellow River water increased the concentration of Na+ in groundwater, which further enhanced the dissolution. Arsenic had a load of 0.728 in factor F3 (redox environment). Arsenic was positively correlated with Fe2+ and NH4+, and negatively correlated with NO3- and SO42-. The lower Eh corresponds to the higher arsenic concentration. The reductive environment in the front alluvial-diluvial depression of Taihang Mountain and the Yellow River flood fan was favorable for the reductive dissolution of arsenic containing iron oxides/hydroxides, resulting in the formation of the high-arsenic groundwater. The desorption of arsenate/arsenite/fluoride in the form of anions on the mineral surface caused by the increase of pH value was favorable for the coexistence of arsenic and fluoride in groundwater. However, the correlation between arsenic and fluoride in groundwater in this area was not significant. The high concentration of calcium ions in the high arsenic region was not conducive to the enrichment of fluoride. In contrast, the weak reducing conditions in the high fluoride region were not conducive to the dissolution of arsenic-containing iron oxides/hydroxides.
CONCLUSIONS The results clarify the coexistence mechanism of arsenic and fluoride in the North Henan Plain, and enrich the theoretical system of co-contamination of groundwater with high arsenic and fluoride.
-

-
表 1 豫北平原浅层地下水水化学特征
Table 1. Summary of hydrogeochemical parameters of groundwater in the North Henan Plain
水化学组分指标 含量平均值(mg/L) 含量最大值(mg/L) 含量最小值(mg/L) 地下水质量分类占比(%) Ⅰ~Ⅲ Ⅳ Ⅴ 总溶解固体(TDS) 1099.05 9374.00 326.00 65.06 27.41 7.53 硫酸根(SO42-) 199.86 4431.00 5.82 81.33 6.93 11.75 亚铁离子(Fe2+) 1.82 13.95 0.01 18.37 49.40 32.23 NH4+(以N计) 0.19 2.70 0.01 90.96 7.23 1.81 NO3-(以N计) 0.689 17.570 0.010 100.00 0.00 0.00 As 0.0101 0.1900 0.0001 75.60 21.09 3.31 F 0.88 4.94 0.13 74.40 17.47 8.13 表 2 水化学指标的因子载荷
Table 2. Factor loadings of hydrogeochemical parameters
水化学指标 因子载荷值 F1 F2 F3 TDS 0.987△ 0.073 -0.078 Mg2+ 0.924△ -0.043 -0.059 Na+ 0.886△ 0.295 -0.106 Cl- 0.877△ -0.117 -0.114 SO42- 0.873△ 0.049 -0.044 Ca2+ 0.732△ -0.524△ 0.010 HCO3- 0.553△ 0.527△ 0.002 F 0.214 0.743△ -0.230 pH -0.161 0.640△ 0.054 NH4+ -0.043 -0.153 0.762△ As -0.068 -0.143 0.728△ Eh 0.053 -0.185 -0.692△ 注:标注“△”的数据表示某变量对该主因子的载荷绝对值大于0.5,为相对重要的变量。 -
[1] Raju N J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies[J]. Environmental Research, 2022, 203: 111782. doi: 10.1016/j.envres.2021.111782
[2] 任宇, 曹文庚, 潘登, 等. 2010—2020年黄河下游河南典型灌区浅层地下水中砷和氟的演化特征及变化机制[J]. 岩矿测试, 2021, 40(6): 846-859. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202110090143
Ren Y, Cao W G, Pan D, et al. Evolution characteristics and change mechanism of arsenic and fluorine in shallow groundwater from a typical irrigation area in the lower reaches of the Yellow River (Henan) in 2010—2020[J]. Rock and Mineral Analysis, 2021, 40(6): 846-859. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202110090143
[3] 袁翰卿, 李巧, 陶洪飞, 等. 新疆奎屯河流域地下水砷富集因素[J]. 环境化学, 2020, 39(2): 524-530. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX202002027.htm
Yuan H Q, Li Q, Tao H F, et al. Groundwater arsenic enrichment factors of Kuitun River Basin, Xinjiang[J]. Environmental Chemistry, 2020, 39(2): 524-530. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX202002027.htm
[4] 张文凯, 于坤, 李勇志, 等. 河套平原地下水环境质量研究综述及展望[J]. 环境化学, 2020, 39(2): 489-499. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX202002024.htm
Zhang W K, Yu K, Li Y Z, et al. Research progress of groundwater environment in Hetao Plain[J]. Environmental Chemistry, 2020, 39(2): 489-499. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX202002024.htm
[5] Cao W G, Guo H M, Zhang Y L, et al. Controls of paleochannels on groundwater arsenic distribution in shallow aquifers of alluvial plain in the Hetao Basin, China[J]. Science of the Total Environment, 2018, 613-614: 958-968. doi: 10.1016/j.scitotenv.2017.09.182
[6] Podgorski J, Berg M. Global threat of arsenic in ground-water[J]. Science, 2020, 368: 845-850. doi: 10.1126/science.aba1510
[7] Parrone D, Ghergo S, Frollini E, et al. Arsenic-fluoride co-contamination in groundwater: Background and anomalies in a volcanic-sedimentary aquifer in central Italy[J]. Journal of Geochemical Exploration, 2020, 217: 106590. doi: 10.1016/j.gexplo.2020.106590
[8] 韩双宝, 李甫成, 王赛, 等. 黄河流域地下水资源状况及其生态环境问题[J]. 中国地质, 2021, 48(4): 1001-1019. https://www.cnki.com.cn/Article/CJFDTOTAL-DIZI202104004.htm
Han S B, Li F C, Wang S, et al. Groundwater resource and eco-environmental problem of the Yellow River Basin[J]. Geology in China, 2021, 48(4): 1001-1019. https://www.cnki.com.cn/Article/CJFDTOTAL-DIZI202104004.htm
[9] Wu R, Podgorski J, Berg M, et al. Geostatistical model of the spatial distribution of arsenic in groundwaters in Gujarat State, India[J]. Environmental Geochemistry and Health, 2021, 43: 2649-2664. doi: 10.1007/s10653-020-00655-7
[10] Bhattacharya P, Claesson M, Bundschuh J, et al. Distribu-tion and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina[J]. Science of the Total Environment, 2006, 358: 97-120. doi: 10.1016/j.scitotenv.2005.04.048
[11] Wang Y X, Li J X, Ma T, et al. Genesis of geogenic contaminated groundwater: As, F and I[J]. Critical Reviews in Environmental Science and Technology, 2021, 51(24): 2895-2933. doi: 10.1080/10643389.2020.1807452
[12] María T A, Jochen B, Bibhash N, et al. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation[J]. Journal of Hazardous Materials, 2013, 262: 960-969. doi: 10.1016/j.jhazmat.2012.08.005
[13] Kumar M, Das A, Das N, et al. Co-occurrence perspective of arsenic and fluoride in the groundwater of Diphu, Assam, northeastern India[J]. Chemosphere, 2016, 150: 227-238. doi: 10.1016/j.chemosphere.2016.02.019
[14] 韩颖, 张宏民, 张永峰, 等. 大同盆地地下水高砷、氟、碘分布规律与成因分析及质量区划[J]. 中国地质调查, 2017, 4(1): 57-68. doi: 10.19388/j.zgdzdc.2017.01.09
Han Y, Zhang H M, Zhang Y F, et al. Distribution regularity, origin and quality division of high arsenic, fluorine and iodine contents in groundwater in Datong Basin[J]. Geological Survey of China, 2017, 4(1): 57-68. doi: 10.19388/j.zgdzdc.2017.01.09
[15] Chen J, Qian H, Wu H, et al. Assessment of arsenic and fluoride pollution in groundwater in Dawukou area, northwest China, and the associated health risk for inhabitants[J]. Environmental Earth Sciences, 2017, 76: 314. doi: 10.1007/s12665-017-6629-2
[16] Kumar M, Goswami R, Patel A K, et al. Scenario, perspectives and mechanism of arsenic and fluoride co-occurrence in the groundwater: A review[J]. Chemosphere, 2020, 249: 126126. doi: 10.1016/j.chemosphere.2020.126126
[17] Duan L, Wang W K, Sun Y B, et al. Hydrogeochemical characteristics and health effects of iodine in groundwater in Wei River Basin[J]. Exposure Health, 2020, 12: 369-383. doi: 10.1007/s12403-020-00348-7
[18] Bondu R, Cloutier V, Rosa E, et al. An exploratory data analysis approach for assessing the sources and distribution of naturally occurring contaminants (F, Ba, Mn, As) in groundwater from southern Quebec (Canada)[J]. Applied Geochemistry, 2020, 114: 104500. doi: 10.1016/j.apgeochem.2019.104500
[19] He X D, Li P Y, Ji Y J, et al. Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: Occurrence, distribution and management[J]. Exposure and Health, 2020, 12: 355-368. doi: 10.1007/s12403-020-00347-8
[20] Zhang Y J, Li J F, Hao Q C, et al. Sources and hydrogeological conditions that cause high iodine concentrations in deep groundwater in the Zhangwei Watershed, North China Plain[J]. Environmental Earth Sciences, 2021, 80: 174. doi: 10.1007/s12665-021-09463-3
[21] Guo H M, Zhang Y, Xing L N, et al. Spatial variation in arsenic and fluoride concentrations of shallow groundwater from the town of Shahai in the Hetao Basin, Inner Mongolia[J]. Applied Geochemistry, 2012, 27: 2187-2196. doi: 10.1016/j.apgeochem.2012.01.016
[22] Kwong H T, Jiao J J, Liu K, et al. Geochemical signature of pore water from core samples and its implications on the origin of saline pore water in Cangzhou, North China Plain[J]. Journal of Geochemical Exploration, 2015, 157: 143-152. doi: 10.1016/j.gexplo.2015.06.008
[23] Li J X, Wang Y T, Zhu C J, et al. Hydrogeochemical processes controlling the mobilization and enrichment of fluoride in groundwater of the North China Plain[J]. Science of the Total Environment, 2020, 730: 138877. doi: 10.1016/j.scitotenv.2020.138877
[24] 何锦, 张福存, 韩双宝, 等. 中国北方高氟地下水分布特征与成因分析[J]. 中国地质, 2010, 37(3): 621-626. doi: 10.3969/j.issn.1000-3657.2010.03.012
He J, Zhang F C, Han S B, et al. The distribution and genetic types of high-fluoride groundwater in northern China[J]. Geology in China, 2010, 37(3): 621-626. doi: 10.3969/j.issn.1000-3657.2010.03.012
[25] Pant N, Rai S P, Singh R, et al. Impact of geology and anthropogenic activities over the water quality with emphasis on fluoride in water scarce Lalitpur district of Bundelkhand region, India[J]. Chemosphere, 2021, 279: 130496. doi: 10.1016/j.chemosphere.2021.130496
[26] Ren X F, Li P Y, He X D, et al. Hydrogeochemical proce-sses affecting groundwater chemistry in the central part of the Guanzhong Basin, China[J]. Archives of Environmental Contamination and Toxicology, 2021, 80: 74-91. doi: 10.1007/s00244-020-00772-5
[27] Nickson R, McArthur J, Burgess W, et al. Arsenic poisoning of Bangladesh groundwater[J]. Nature, 1998, 395: 338. doi: 10.1038/26387
[28] 董会军, 董建芳, 王昕洲, 等. pH值对HPLC-ICP-MS测定水体中不同形态砷化合物的影响[J]. 岩矿测试, 2019, 38(5): 510-517. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.201808230096
Dong H J, Dong J F, Wang X Z, et al. Effect of pH on determination of various arsenic species in water by HPLC-ICP-MS[J]. Rock and Mineral Analysis, 2019, 38(5): 510-517. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.201808230096
[29] 余倩, 张宇, 邬建勋, 等. 江汉平原沉积物中磷酸盐与砷的竞争吸附机制[J]. 中南民族大学学报(自然科学版), 2020, 39(4): 337-342. https://www.cnki.com.cn/Article/CJFDTOTAL-ZNZK202004002.htm
Yu Q, Zhang Y, Wu J X, et al. Competitive adsorption mechanism of phosphate and arsenic in sediments from Jianghan Plain[J]. Journal of South-Central University for Nationalities (Natural Science Edition), 2020, 39(4): 337-342. https://www.cnki.com.cn/Article/CJFDTOTAL-ZNZK202004002.htm
[30] 潘敖然, 单慧媚, 彭三曦, 等. 基于热力学模拟河套平原高砷地下水中硫代砷形态分布特征[J]. 地球科学进展, 2018, 33(11): 1169-1180. doi: 10.11867/j.issn.1001-8166.2018.11.1169.
Pan A R, Shan H M, Peng S X, et al. Thermodynamic modeling of thioarsenic species distribution in high As groundwater in Hetao Plain[J]. Advances in Earth Science, 2018, 33(11): 1169-1180. doi: 10.11867/j.issn.1001-8166.2018.11.1169.
[31] Deng Y M, Zheng T L, Wang Y X, et al. Effect of microbially mediated iron mineral transformation on temporal variation of arsenic in the Pleistocene aquifers of the central Yangtze River Basin[J]. Science of the Total Environment, 2018, 619-620: 1247-1258. doi: 10.1016/j.scitotenv.2017.11.166
[32] 吕晓立, 刘景涛, 朱亮, 等. 甘肃省秦王川盆地地下水氟富集特征及影响因素[J]. 干旱区资源与环境, 2020, 34(3): 188-195. https://www.cnki.com.cn/Article/CJFDTOTAL-GHZH202003027.htm
Lyu X L, Liu J T, Zhu L, et al. Evolution feature and gensis of fluoride groundwater in shallow aquifer from Qin Wangchuan Basin[J]. Journal of Arid Land Resources and Environment, 2020, 34(3): 188-195. https://www.cnki.com.cn/Article/CJFDTOTAL-GHZH202003027.htm
[33] Li J X, Zhou H L, Qian K, et al. Fluoride and iodine enrichment in groundwater of North China Plain: Evidences from speciation analysis and geochemical modeling[J]. Science of the Total Environment, 2017, 598: 239-248. doi: 10.1016/j.scitotenv.2017.04.158
[34] Shen M M, Guo H M, Jia Y F, et al. Partitioning and reactivity of iron oxide minerals in aquifer sediments hosting high arsenic groundwater from the Hetao Basin, P.R. China[J]. Applied Geochemistry, 2018, 89: 190-201.
[35] 吴昆明, 郭华明, 魏朝俊. 改性磁铁矿对水体中砷的吸附特性研究[J]. 岩矿测试, 2017, 36(6): 624-632. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.201709110147
Wu K M, Guo H M, Wei C J. Adsorption characteristics of arsenic in water by modified magnetite[J]. Rock and Mineral Analysis, 2017, 36(6): 624-632. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.201709110147
[36] Smith R L, Kent D B, Repert D A, et al. Anoxic nitrate reduction coupled with iron oxidation and attenuation of dissolved arsenic and phosphate in a sand and gravel aquifer[J]. Geochimica et Cosmochimica Acta, 2017, 196: 102-120.
[37] 李谨丞, 曹文庚, 潘登, 等. 黄河冲积扇平原浅层地下水中氮循环对砷迁移富集的影响[J]. 岩矿测试, 2022, 41(1): 120-132. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202110080140
Li J C, Cao W G, Pan D, et al. Influences of nitrogen cycle on arsenic enrichment in shallow groundwater from the Yellow River Alluvial Fan Plain[J]. Rock and Mineral Analysis, 2022, 41(1): 120-132. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202110080140
[38] 曹文庚, 董秋瑶, 谭俊, 等. 河套盆地晚更新世以来黄河改道对高砷地下水分布的控制机制[J]. 南水北调与水利科技, 2021, 19(1): 140-150. https://www.cnki.com.cn/Article/CJFDTOTAL-NSBD202101014.htm
Cao W G, Dong Q Y, Tan J, et al. Mechanism of Yellow River diversion in controlling high arsenic groundwater distribution since Late Pleistocene[J]. South-to-North Water Transfers and Water Science & Technology, 2021, 19(1): 140-150. https://www.cnki.com.cn/Article/CJFDTOTAL-NSBD202101014.htm
-



 下载:
下载: