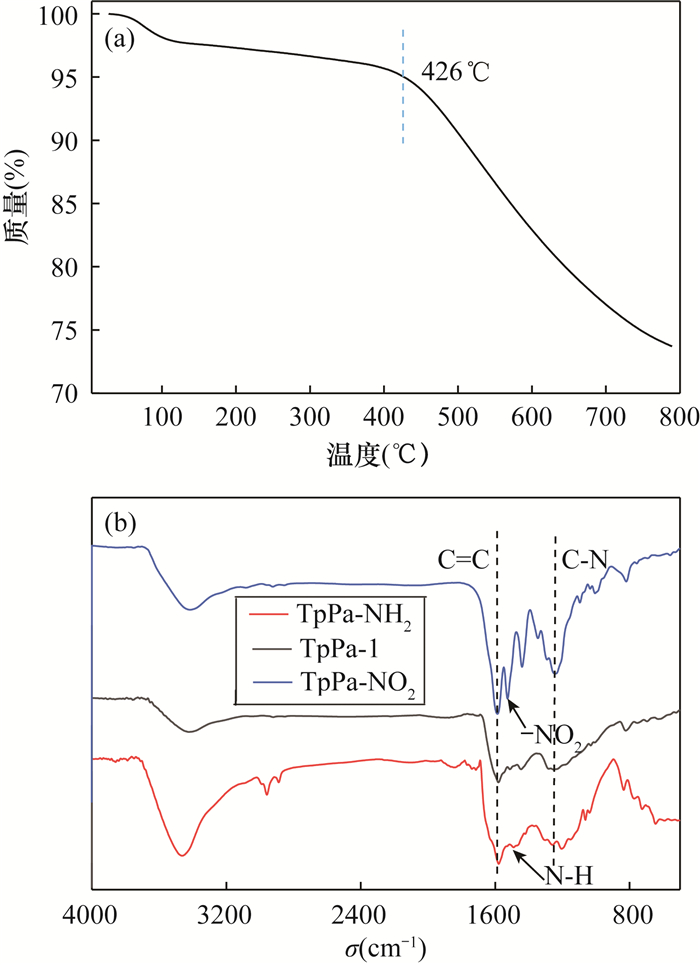
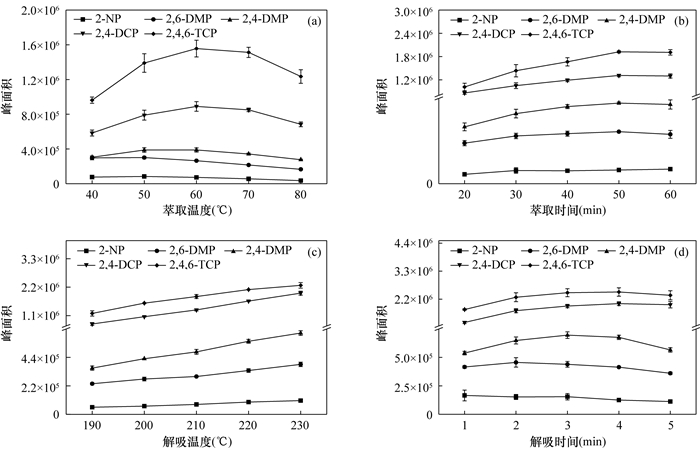
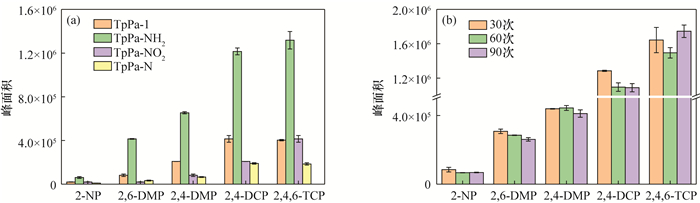
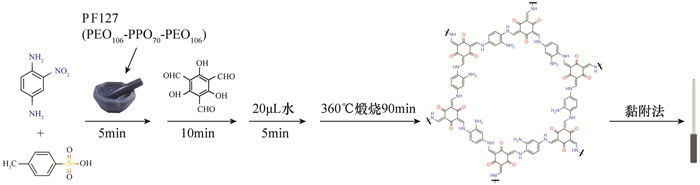
An Amino-functionalized Covalent Organic Framework Coating for Highly Efficient Solid Phase Microextraction of Trace Phenols in Water
-
摘要:
酚类化合物是一类常见的环境污染物,由于浓度低、极性较强且样品基质复杂,对其分析检测前需采用样品前处理技术以进行有效地分离和富集。固相微萃取(SPME)是一种集采样、富集、进样于一体的无溶剂前处理技术,与气相色谱-质谱(GC-MS)等联用可实现复杂基质中痕量有机物的快速富集和检测。本文采用无溶剂合成策略,一步合成了氨基改性的共价有机骨架(COFs)材料(TpPa-NH2),合成方法简单绿色,无需溶剂。将其制备成SPME涂层,以5种酚类化合物作为目标分析物,基于顶空模式进行萃取,结合GC-MS作为检测手段,建立了SPME-GC-MS检测酚类化合物的新方法。与未氨基改性的TpPa-1涂层相比,TpPa-NH2萃取酚类化合物的性能是其3~5倍,表明氨基官能团可有效地提高萃取酚类化合物的性能。在最佳条件下,该方法线性范围为10~5.0×104ng/L,线性相关系数为0.996~0.999,检出限为1.30~5.35ng/L。涂层批内的相对标准偏差(RSD)在4.2%~8.9%之间,批间的RSD在2.6%~8.2%之间,且该涂层可以重复使用90次以上。基于TpPa-NH2涂层材料建立的SPME-GC-MS检测酚类化合物的分析方法,已成功应用于环境水样标准物质中酚类化合物的检测。
Abstract:BACKGROUND Phenolic compounds, as ubiquitous pollutants, should be effectively separated by sample pretreatment technology prior to analysis because of the low concentration, strong polarity and complex sample matrix. Solid phase microextraction (SPME) is a solvent-free pretreatment technology integrating sampling, enrichment and injection. Combined with gas chromatography-mass spectrometry (GC-MS), it can achieve the rapid enrichment and detection of trace organic compounds in a complex matrix.
OBJECTIVES To develop a sensitive, simple, and environmentally friendly method for the determination of trace phenols.
METHODS An amino functionalized covalent organic framework (TpPa-NH2) was synthesized by a solvent-free method in one step. SPME was used as the coating, four kinds of phenolic compounds were used as target analytes, and a new method for the detection of phenolic compounds was established by headspace extraction mode combined with GC-MS.
RESULTS The extraction performance of TpPa-NH2 was 3-5 times that of TpPa-1. Under the optimum conditions, the established analysis method for four phenols had wide linear ranges (10-5.0×104ng/L), high linear correlation coefficients (0.996-0.999), and low detection limits (1.30-5.35ng/L). Both the intra-fiber repeatability (RSD from 2.2%-9.2%) and inter-fiber reproducibility (RSD from 4.2%-8.9%) were satisfactory, and the coating can be reused more than 90 times.
CONCLUSIONS The introduction of an amino group can effectively improve the extraction performance of TpPa for phenolic compounds. The established method establishes the sensitive, convenient and green detection of phenolic compounds in actual samples, demonstrating a good application prospect.
-

-
表 1 TpPa-NH2涂层萃取5种酚类化合物的分析性能
Table 1. Analysis performance of 5 kinds of phenolic compounds by TpPa-NH2 coating
酚类化合物 线性范围
(ng/L)R2 检出限
(ng/L)RSD(%) 批内重复性(n=3) 批间重复性(n=3) 2-NP 20~5.0×104 0.998 4.64 7.1 2.6 2,4-DMP 10~5.0×104 0.996 1.81 4.2 8.2 2,6-DMP 10~5.0×104 0.997 1.30 7.1 8.1 2,4-DCP 20~5.0×104 0.996 5.35 5.7 5.4 2,4,6-TCP 20~5.0×104 0.999 4.59 8.9 3.4 表 2 与已报道的基于SPME-GC-MS检测酚类的材料对比
Table 2. Comparison with the reported materials for the detection of phenols based on SPME-GC-MS
表 3 本文方法与国家标准方法检测酚类化合物结果对比
Table 3. Comparison of results with the study method and national standard methods for detection of the phenols
酚类化合物检测方法 进样量 检出限 《水质酚类化合物的测定液液萃取法/气相色谱法》 (HJ 676—2013) 500mL 0.5~3.4μg/L 《固体废物酚类化合物的测定气相色谱法》(HJ 711—2014) 100mL 2~6μg/L 本文方法 10.0μL 1.30~5.35ng/L 表 4 标准物质分析结果
Table 4. Analytical results of the reference materials
标准品 酚类化合物 参考值
(μg/L)测定值
(μg/L)RSD(%)
(n=5)BWQ8341—2016 2-NP 5±0.15 5.10±0.00 0.04 2,4-DMP 5±0.15 4.95±0.05 0.92 2,4-DCP 5±0.15 5.37±0.04 0.83 2,4,6-TCP 5±0.15 5.04±0.15 2.90 BWQ8236—2016 2-NP 5±0.15 5.09±0.19 3.90 2,4-DMP 5±0.15 4.93±0.02 0.44 2,4-DCP 5±0.15 5.36±0.04 0.87 2,4,6-TCP 5±0.15 4.98±0.08 1.60 -
[1] Sas O G, Sanchez P B, Gonzalez B, et al. Removal of phenolic pollutants from wastewater streams using ionic liquids[J]. Separation and Purification Technology, 2019, 236: 116310.
[2] 李忠煜, 李艳广, 黎卫亮, 等. 衍生化气相色谱-质谱法测定复垦土地样品中19种酚类污染物[J]. 岩矿测试, 2021, 40(2): 239-249. doi: 10.15898/j.cnki.11-2131/td.202007080101 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202007080101
Li Z Y, Li Y G, Li W L, et al. Determination of 19 phenolic pollutants in reclaimed land samples by derivation gas chromatography-mass spectrometry[J]. Rock and Mineral Analysis, 2021, 40(2): 239-249. doi: 10.15898/j.cnki.11-2131/td.202007080101 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202007080101
[3] Khan A S, Ibrahim T H, Jabbar N A, et al. Ionic liquids and deep eutectic solvents for the recovery of phenolic compounds: Effect of ionic liquids structure and process parameters[J]. RSC Advances, 2021, 11(20): 12398-12422. doi: 10.1039/D0RA10560K
[4] Ramos R L, Martins M F, Lebron Y A R, et al. Membrane distillation process for phenolic compounds removal from surface water[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105588. doi: 10.1016/j.jece.2021.105588
[5] Castro M D, Priego-Capote F. Soxhlet extraction: Past and present panacea[J]. Journal of Chromatography A, 2010, 1217(16): 2383-2389. doi: 10.1016/j.chroma.2009.11.027
[6] Belo R, Figueiredob J P, Nunes C M, et al. Accelerated solvent extraction method for the quantification of polycyclic aromatic hydrocarbons in cocoa beans by gas chromatography-mass spectrometry[J]. Journal of Chromatography B, 2017, 1053: 87-100. doi: 10.1016/j.jchromb.2017.03.017
[7] 孙书堂, 严倩, 黎宁, 等. 铁丝原位自转化-固相微萃取新涂层应用于萃取环境水样中多环芳烃的性能研究[J]. 岩矿测试, 2020, 39(3): 408-416. doi: 10.15898/j.cnki.11-2131/td.202002030014 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202002030014
Sun S T, Yan Q, Li N, et al. In situ self-transforming membrane as solid phase microextraction coating extraction of PAHs in environmental water samples[J]. Rock and Mineral Analysis, 2020, 39(3): 408-416. doi: 10.15898/j.cnki.11-2131/td.202002030014 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202002030014
[8] 熊茂富, 任敏, 杜伊, 等. 顶空固相微萃取-气相色谱-质谱联用法同时测定湖库水中12种氯苯甲醚的条件优化[J]. 岩矿测试, 2019, 38(6): 724-733. doi: 10.15898/j.cnki.11-2131/td.201901210016 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.201901210016
Xiong M F, Ren M, Du Y, et al. Simultaneous determination of 12 chloroanisoles in lake reservoir waters by headspace solid phase microextraction-gas chromatography-mass spectrometry[J]. Rock and Mineral Analysis, 2019, 38(6): 724-733. doi: 10.15898/j.cnki.11-2131/td.201901210016 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.201901210016
[9] Allafchian A R, Majidian Z, Ielbeigi V, et al. A novel method for the determination of three volatile organic compounds in exhaled breath by solid-phase microextraction-ion mobility spectrometry[J]. Analytical and Bioanalytical Chemistry, 2016, 408(3): 839-847. doi: 10.1007/s00216-015-9170-8
[10] Jiang H, Li J S, Jiang M Y, et al. Ordered mesoporous carbon film as an effective solid-phase microextraction coating for determination of benzene series from aqueous media[J]. Analytica Chimica Acta, 2015, 888: 85-93. doi: 10.1016/j.aca.2015.06.055
[11] Zhou X Q, Xie Y L, Zhao Z D, et al. A simple strategy based on fibers coated with surfactant-functionalized multiwalled carbon nanotubes to improve the properties of solid-phase microextraction of phenols in aqueous solution[J]. BMC Chemistry, 2020, 14(1): 15. doi: 10.1186/s13065-020-00665-7
[12] Feng J J, Feng J Q, Ji X P, et al. Recent advances of covalent organic frameworks for solid-phase microextraction[J]. Trends in Analytical Chemistry, 2021, 137: 116208. doi: 10.1016/j.trac.2021.116208
[13] Hou X D, Wang L C, Guo Y. Recent developments in solid-phase microextraction coatings for environmental and biological analysis[J]. Chemistry Letters, 2017, 46(10): 1444-1455. doi: 10.1246/cl.170366
[14] Khataei M M, Yamini Y, Shamsayei M. Applications of porous frameworks in solid phase microextraction[J]. Journal of Separation Science, 2021, 44(6): 1231-1263. doi: 10.1002/jssc.202001172
[15] Aziz-Zanjani M O, Mehdinia A. A review on procedures for the preparation of coatings for solid phase microextraction[J]. Microchimica Acta, 2014, 181(11-12): 1169-1190. doi: 10.1007/s00604-014-1265-y
[16] Geng K Y, He T, Liu R Y, et al. Covalent organic frame-works: Design, synthesis, and functions[J]. Chemical Reviews, 2020, 120(16): 8814-8933. doi: 10.1021/acs.chemrev.9b00550
[17] Lohse M S, Bein T. Covalent organic frameworks: Stru-ctures, synthesis, and applications[J]. Advanced Functional Materials, 2018, 28(33): 1705553. doi: 10.1002/adfm.201705553
[18] Huang X, Sun C, Feng X. Crystallinity and stability of covalent organic frameworks[J]. Science China(Chemistry), 2020, 63(10): 1367-1390.
[19] Li Y J, Wang H J, Zhao W F, et al. Facile synthesis of a triptycene-based porous organic polymer with a high efficiency and recyclable adsorption for organic dyes[J]. Journal of Applied Polymer Science, 2019, 136(39): 47987. doi: 10.1002/app.47987
[20] Yang Q, Luo M L, Liu K W, et al. Covalent organic frameworks for photocatalytic applications[J]. Applied Catalysis B: Environmental, 2020, 276: 119174. doi: 10.1016/j.apcatb.2020.119174
[21] Cheng H Y, Wang T. Covalent organic frameworks in catalytic organic synthesis[J]. Advanced Synthesis & Catalysis, 2020, 363(1): 144-193.
[22] Yu G, Wang C. Research progress of covalent organic frameworks in sensing[J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1437-1447. doi: 10.6023/cjoc202003018
[23] Skorjanc T, Shetty D, Valant M. Covalent organic polymers and frameworks for fluorescence-based sensors[J]. ACS Sensors, 2021, 6(4): 1461-1481. doi: 10.1021/acssensors.1c00183
[24] Qian H L, Yang C X, Wang W L, et al. Advances in covalent organic frameworks in separation science[J]. Journal of Chromatography A, 2018, 1542: 1-18. doi: 10.1016/j.chroma.2018.02.023
[25] Das S, Feng J, Wang W. Covalent organic frameworks in separation[J]. Annual Review of Chemical and Biomolecular Engineering, 2020, 11: 131-153. doi: 10.1146/annurev-chembioeng-112019-084830
[26] Zhu D Y, Xu G Y, Barnes M, et al. Covalent organic frameworks for batteries[J]. Advanced Functional Materials, 2021, 31(32): 2100505. doi: 10.1002/adfm.202100505
[27] Huo J Q, Luo B C, Chen Y. Crystalline covalent organic frameworks from triazine nodes as porous adsorbents for dye pollutants[J]. ACS Omega, 2019, 4(27): 22504-22513. doi: 10.1021/acsomega.9b03176
[28] Liu L, Meng W K, Li L, et al. Facile room-temperature synthesis of a spherical mesoporous covalent organic framework for ultrasensitive solid-phase microextraction of phenols prior to gas chromatography-tandem mass spectrometry[J]. Chemical Engineering Journal, 2019, 369: 920-927. doi: 10.1016/j.cej.2019.03.148
[29] Lv G, Liu J, Xiong Z, et al. Selectivity adsorptive mechanism of different nitrophenols on UiO-66 and UiO-66-NH2 in aqueous solution[J]. Journal of Chemical & Engineering Data, 2016, 61(11): 3868-3876.
[30] Ma T T, Shen X F, Yang C, et al. Covalent immobilization of covalent organic framework on stainless steel wire for solid-phase microextraction GC-MS/MS determination of sixteen polycyclic aromatic hydrocarbons in grilled meat samples[J]. Talanta, 2019, 201: 413-418. doi: 10.1016/j.talanta.2019.04.031
[31] Wu T, Zang X H, Wang M T, et al. Covalent organic framework as fiber coating for solid-phase microextraction of chlorophenols followed by quantification with gas chromatography-mass spectrometry[J]. Journal of Agricultural and Food Chemistry, 2018, 66(42): 11158-11165. doi: 10.1021/acs.jafc.8b01643
[32] Jin D, Wang B W, Wu X C, et al. Construction and catalytic applications of an amino-functionalized covalent organic framework[J]. Transition Metal Chemistry, 2019, 44(8): 689-697. doi: 10.1007/s11243-019-00335-1
[33] Jiang Y Z, Liu C Y, Huang A S, et al. EDTA-functiona-lized covalent organic framework for the removal of heavy-metal ions[J]. ACS Applied Materials & Interfaces, 2019, 11(35): 32186-32191.
[34] Wang K X, Yang L P, Li H X, et al. Surfactant pyrolysis-guided in situ fabrication of primary amine-rich ordered mesoporous phenolic resin displaying efficient heavy metal removal[J]. ACS Applied Materials & Interfaces, 2019, 11(24): 21815-21821.
[35] Zanganeh F, Yamini Y, Khataei M M, et al. Ethane-bridge periodic mesoporous organosilica materials as a novel fiber coating in headspace solid-phase microextraction of phthalate esters from saliva and PET container samples[J]. Analytical and Bioanalytical Chemistry, 2022, 414(6): 2285-2296. doi: 10.1007/s00216-021-03868-6
[36] Chandra S, Kandambeth S, Biswal B, et al. Chemically stable multilayered covalent organic nanosheets from covalent organic frameworks via mechanical delamination[J]. Journal of the American Chemical Society, 2013, 135(47): 17853-17861. doi: 10.1021/ja408121p
[37] Wang W C, Wang J T, Zhang S H, et al. A novel Schiff base network-1 nanocomposite coated fiber for solid-phase microextraction of phenols from honey samples[J]. Talanta, 2016, 161: 22-30. doi: 10.1016/j.talanta.2016.08.009
[38] Tafazoli Z, Liu L, Azar P A, Tehrani M S, et al. Facile preparation of multifunctional carbon nanotube/magnetite/polyaniline nanocomposite offering a strong option for efficient solid-phase microextraction coupled with GC-MS for the analysis of phenolic compounds[J]. Journal of Separation Science, 2018, 41(13): 2736-2642. doi: 10.1002/jssc.201800062
[39] Liu Y, Huang Y F, Chen G S, et al. A graphene oxide-based polymer composite coating for highly-efficient solid phase microextraction of phenols[J]. Analytica Chimica Acta, 2018, 1015: 20-26. doi: 10.1016/j.aca.2018.02.034
-



 下载:
下载: