Comparative Analysis of Spectral Characteristics and Color Origin of Chameleon Diamond and Similar Yellow Diamond
-
摘要:
Chameleon钻石(俗称“变色龙钻石”)是一种具有光致变色和热致变色现象的彩色钻石,在受热后或长时间在暗室中会由黄绿色变为黄色,在实际工作中发现与Chameleon钻石颜色相似的黄色钻石并不具有此类“变色”现象。为了研究Chameleon钻石与相似黄色钻石的差异,并进一步探究产生光致变色和热致变色现象的原因,本文选取Chameleon钻石和与其颜色相似的黄色钻石作为研究对象,进行加热实验,观察加热前后钻石颜色、紫外可见光吸收光谱的变化情况,探寻导致热致变色的原因,并对样品的红外吸收光谱及光致发光光谱特征进行详细分析与比较。结果表明:Chameleon钻石受热后由黄绿色变为黄色,在超短波紫外线下显示绿色荧光和磷光,相似黄色钻石样品无磷光及变色现象;所有样品均有从紫外光至510nm逐渐减弱的连续吸收和480nm吸收宽带,Chameleon钻石具650~800nm吸收宽带,相似黄色钻石无该吸收带,加热后吸收带消失是其产生热致变色的主要原因;进一步对样品的缺陷类型进行对比分析,Chameleon钻石红外光谱可见孤氮特征及与片晶无关的1430cm−1宽吸收带,该峰未在相似黄色钻石中出现,但产生该吸收的原因未知,仍需进一步研究,此外与Chameleon钻石相比,相似黄色钻石中氮杂质的聚合程度更高;Chameleon钻石及相似黄色钻石表现出以700nm为中心的特征发光带,此外Chameleon钻石还可检测到H3等与N-V相关缺陷及489、883、884nm等与Ni-N相关缺陷产生的发光峰。综合以上光谱学特征对比分析可知,Chameleon钻石存在与A型氮、孤氮、镍杂质及氢杂质有关的某种缺陷结构,与相似黄色钻石的光谱特征具有明显差异。本次研究对探明Chameleon钻石具热致变色现象的根本原因具有参考意义。
Abstract:BACKGROUND Chameleon diamond is a type of color diamond with photochromic and thermochromic phenomenon. If the chameleon diamond is placed in a dark room or heated for a long time, it can change from greenish yellow to fancy yellow, and return to its original color when it cools. Previous studies on chameleon diamond focus mainly on spectral characteristics. Through testing a large number of samples, the gemological and spectral characteristics of chameleon diamond are summarized. The characteristics of chameleon diamond, include a persistent yellow phosphorescence, a 480nm absorption band, and mainly the A-aggregate of nitrogen. A model to explain the thermochromic and photochromic phenomenon of chameleon diamond was proposed. The characteristics of chameleon diamond discovered by previous studies are also found in a few yellow diamonds. Diamonds colored by the 480nm band show yellow fluorescence and phosphorescence under short wave ultraviolet light, low nitrogen content, high concentrations of defects related to hydrogen and nickel, but no obvious thermochromic and photochromic phenomena. Previous studies of the chameleon diamond and similar yellow diamonds were conducted independently and did not compare the two similar diamonds.
OBJECTIVES To ascertain the difference of spectral characteristics and defect types between chameleon diamonds and similar yellow diamonds, and then analyze the causes of thermochromic phenomenon of chameleon diamond to gain a deeper understanding of the possible structure of the center responsible for the chameleon effect.
METHODS Firstly, two chameleon diamond samples and four similar yellow diamond samples were collected to observe the color changes of the diamonds before and after heating. Top illumination and scattering illumination were used to observe the internal characteristics and color distribution of the samples. The fluorescence and phosphorescence characteristics of diamonds at long wave (365nm) and short wave (254nm) were observed by ordinary fluorescent lamp. The fluorescence and phosphorescent characteristics and growth structure of the samples under ultra-violet light were observed by DiamondViewTM. Secondly, in order to investigate the causes of thermochromism in chameleon diamond, the absorption characteristics of diamond samples in the range of ultraviolet visible light were collected before and after heating and compared with those of similar yellow diamonds. The differences in absorption spectra between chameleon diamond and similar yellow diamond were analyzed, and the causes of thermochromic phenomena were explored. Finally, an infrared spectrometer was used to collect the infrared spectrum of diamond samples and analysis of the types and impurity elements of diamond samples was conducted. Through the comparative analysis of the infrared spectrum of samples, the differences in the types and contents of nitrogen and hydrogen impurities between chameleon diamond and similar yellow diamond were obtained. The laser Raman spectrometer was used to collect PL spectra of the samples at liquid nitrogen temperature by using 473nm, 532nm and 830nm lasers. By comparing the PL characteristics of chameleon diamond and similar yellow diamond, the differences between the two defects were obtained.
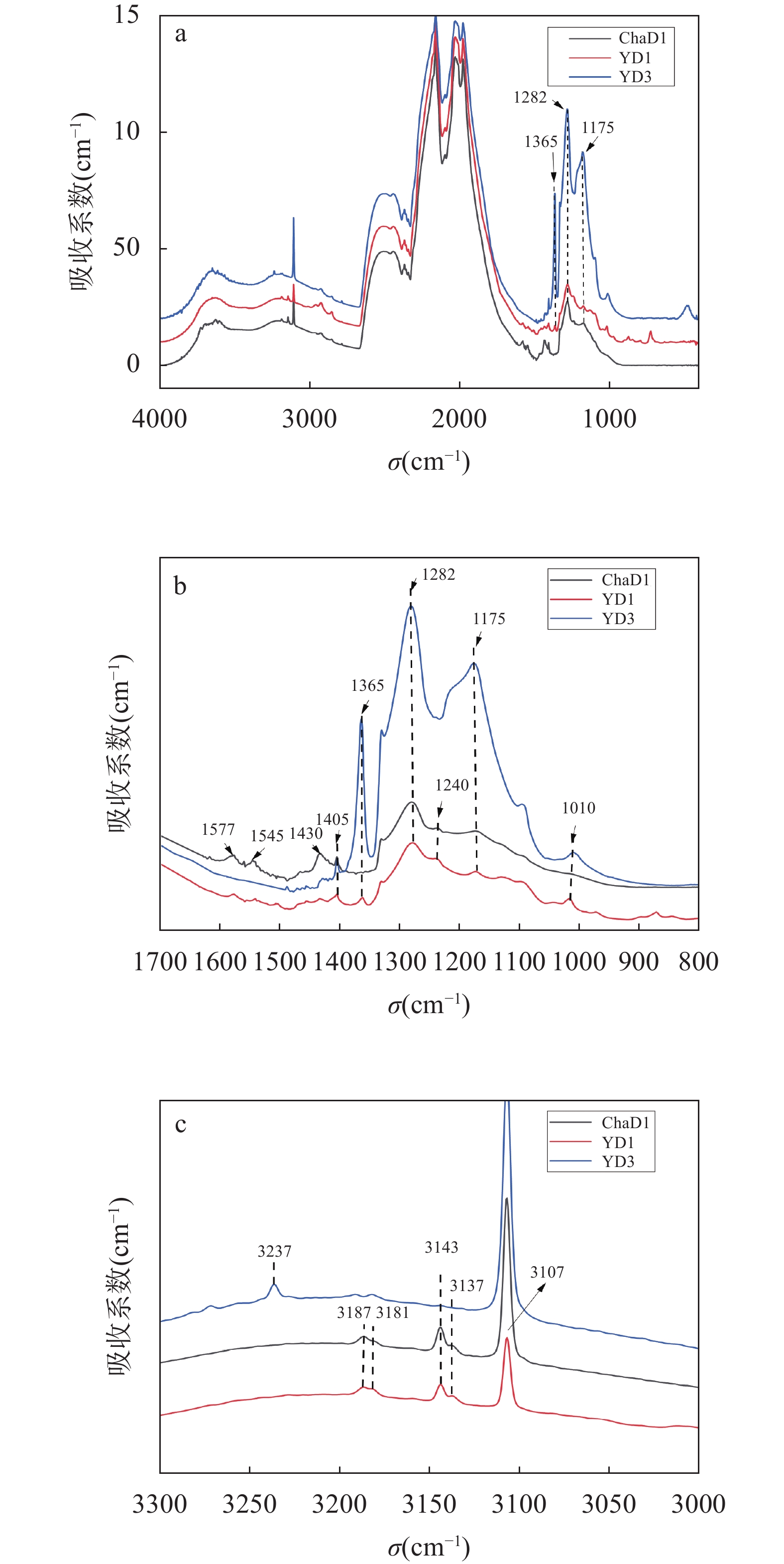
RESULTS The color distribution of the 6 diamond samples is uniform. After heating, the chameleon diamond samples change color from yellow-green to yellow, while similar yellow diamonds have no obvious color change. Under long and short wavelength UV light, the samples are yellow fluorescence without phosphorescence. The performance of the samples in this study is consistent under long and short wavelength ultraviolet light, but it is different from the previous study results that the chameleon diamond has persistent yellow phosphorescence, which further indicates that the chameleon effect is not directly related to the phosphorescence. Under ultra-violet light, the samples have green fluorescence with irregular patterns which are markedly similar to diamonds colored by the 480nm band. Chameleon diamonds show moderate green phosphorescence. All the samples have C defect absorption continuum combined with 480nm absorption band. The specific wide absorption band of 650-800nm is obvious in chameleon diamond. After being heated, the 480nm and 650-800nm absorption band of chameleon diamond completely disappears. However, the 480nm absorption band of similar yellow diamonds is weakened or has no obvious change. Therefore, the poor thermal stability of 480nm and 650-800nm absorption band is responsible for the thermochromic phenomenon of chameleon diamonds. The infrared absorption characteristics of chameleon diamond mainly show A-defect absorption. Isolated nitrogen and hydrogen-related features can be seen, in addition to the characteristic 1430cm−1 wide absorption, which has a different origin than the platelet-related 1430cm−1 feature in Cape diamond. The similar yellow diamond has higher nitrogen content, and the degree of nitrogen aggregation is higher, and does not show any type Ib character. The broadband emission of all samples occurs mainly as one band centered at 700nm, this band consists of a dozen of peaks from 595nm to 725nm in steps of about 10nm. Additionally sharp peaks at 753, 771, 799, 818, 838, 845nm are present in the chameleon diamond samples. This spectrum is virtually identical to that reported previously for diamonds containing the 480nm absorption band. Chameleon diamonds and similar yellow diamonds all contain nitrogen-vacancy, nickel-nitrogen related defects, but the amount of defects is different. Chameleon diamonds cannot be effectively distinguished based on the PL spectral. The PL spectrum analysis shows that the nickel impurity is responsible for the green fluorescence of the samples under ultra-violet light.
CONCLUSIONS This study focuses on the comparative analysis of gemology, UV-visible absorption, infrared absorption and photoluminescence characteristics of chameleon diamond and its similar yellow diamond. Chameleon diamond is type Ia diamond with low concentration of A defect, and shows some type Ib character, such as 1240cm−1 absorption. Chameleon diamond has 480nm and 650-800nm absorption bands, that are responsible for the chameleon effect. The characteristics of chameleon diamond in this study are consistent with those in previous studies. Absorption bands of 650-800nm and absorption peaks of 1430cm−1 (unrelated to platelet) are the main differences between the chameleon diamond and similar yellow diamond sample. It is inferred that the absorption band of 650-800nm and the absorption peak of 1430cm−1 are related to the center causing the thermochromic phenomenon. Although similar yellow diamond samples also have a 480nm center, their thermal stability is different from that of chameleon diamonds. The reason for this phenomenon is unknown, therefore further studies are needed.
-

-
[1] Collins A T. Colour centres in diamond[J]. Journal of Gemmology, 1982, 18(1):37−75. doi: 10.15506/JoG.1982.18.1.37
[2] 宋中华,陆太进,苏隽,等. 光致变色CVD合成钻石的特征[J]. 宝石和宝石学杂志,2016,18(1):1−5. doi: 10.15964/j.cnki.027jgg.2016.01.001
Song Z H, Lu T J, Su J, et al. Silicon-doped CVD synthetic diamonds with photochromic effect[J]. Journal of Gems and Gemmology, 2016, 18(1):1−5. doi: 10.15964/j.cnki.027jgg.2016.01.001
[3] Breeding C M,Shen A. Pink diamonds with a temporary color change[J]. Gems & Gemology, 2005, 41(4):342−344.
[4] Hainschwang T,Simic D,Fritsch E,et al. A gemological study of a collection of chameleon diamonds[J]. Gems & Gemology, 2005, 41(1):20−34.
[5] 蒙宇飞,彭明生,苑执中. 变色金刚石的谱学研究[J]. 矿物学报,2005,25(1):65−68. doi: 10.16461/j.cnki.1000-4734.2005.01.012
Meng Y F,Peng M S,Yuan Z Z. Spectroscopic studies on color-change diamonds[J]. Acta Mineralogica Sinica, 2005, 25(1):65−68. doi: 10.16461/j.cnki.1000-4734.2005.01.012
[6] Fritsch E,Massi L,Rossman G R,et al. Thermochromic and photochromic behavior of “chameleon” diamonds[J]. Diamond & Related Materials, 2007, 16:401−408.
[7] Fritsch E,Delaunay A. What truly characterises a chameleon diamond? An example of an atypical 25.85ct stone[J]. The Journal of Gemmology, 2018, 36(2):142−151. doi: 10.15506/JoG.2018.36.2.142
[8] Ardon T. Chameleon diamond with nickel absorption band[J]. Gems & Gemology, 2014, 50(2):161−162.
[9] Breeding C M,Eaton-Magaa S,Shigley J E. Naturally colored yellow and orange gem diamonds:The nitrogen factor[J]. Gems & Gemology, 2020, 56(2):194.
[10] Fisher D,Sibley S J,Kelly C J. Brown color in natural diamond and interaction between the brown related and other color-inducing defects[J]. Journal of Physics:Condensed Matter, 2009, 21(36):364213. doi: 10.1088/0953-8984/21/36/364213
[11] 唐诗,苏隽,陆太进,等. 化学气相沉积法再生钻石的实验室检测特征研究[J]. 岩矿测试,2019,38(1):62−70. doi: 10.15898/j.cnki.11-2131/td.201802070017
Tang S,Su J,Lu T J,et al. Research on laboratory testing features of chemical vapor deposition in overgrowth diamonds[J]. Rock and Mineral Analysis, 2019, 38(1):62−70. doi: 10.15898/j.cnki.11-2131/td.201802070017
[12] 宋中华, 魏华, 田晶. 钻石辨假[M]. 北京: 文化出版社, 2017.
Song Z H, Wei H, Tian J. Identification of diamonds[M]. Beijing: Cultural Development Press, 2017.
[13] Breeding C M,Eaton-Magaa S,Shigley J E,et al. Natural-color green diamonds:A beautiful conundrum[J]. Gems & Gemology, 2018, 54(1):2−27.
[14] Moses T M,Reinitz I M,Johnson M L,et al. A contribution to understanding the effect of blue fluorescence on the appearance of diamonds[J]. Gems & Gemology, 1997, 33(4):244−259.
[15] Jones R,Goss J P,Briddon P R. Acceptor level of nitrogen in diamond and the 270nm absorption band[J]. Physical Review B:Condensed Matter, 2009, 80(3):1132−1136.
[16] Collins A T. The color of diamond and how it may be changed[J]. Journal of Gemmology, 2001, 27(6):341−359. doi: 10.15506/JoG.2001.27.6.341
[17] Reinitz I M,Buerki P R,Shigley J E,et al. Identification of HPHT-treated yellow to green diamonds[J]. Gems & Gemology, 2000, 36(2):128−137.
[18] Gali A,Lowther J E,Deák P. Defect states of substitutional oxygen in diamond[J]. Journal of Physics:Condensed Matter, 2001, 13(50):11607−11613. doi: 10.1088/0953-8984/13/50/319
[19] Hainschwang T,Notari F,Pamies G. A defect study and classification of brown diamonds with deformation-related color[J]. Minerals, 2020, 10(10):903. doi: 10.3390/min10100903
[20] 杨志军,彭明生,谢先德,等. 金刚石的微区显微红外光谱分析及其意义[J]. 岩矿测试,2002,21(3):161−165. doi: 10.3969/j.issn.0254-5357.2002.03.001
Yang Z J,Peng M S,Xie X D,et al. Micro area analysis of diamond by micro-infrared spectrometry and its significance[J]. Rock and Mineral Analysis, 2002, 21(3):161−165. doi: 10.3969/j.issn.0254-5357.2002.03.001
[21] Fritsch E,Scarratt K. Natural-color nonconductive gray-to-blue diamond[J]. Gems & Gemology, 1992, 28(1):35−42.
[22] Fritsch E. Gemmological properties of type Ia diamonds with an unusually high hydrogen content[J]. The Journal of Gemmology, 1993, 23(8):451−460. doi: 10.15506/JoG.1993.23.8.451
[23] Woods G S. Platelets and the infrared absorption of type Ia diamonds[J]. Proceedings of the Royal Society A, 1986, 407(1832):219−238.
[24] Collins A T,Mohammed K. Optical studies of vibronic bands in yellow luminescing natural diamonds[J]. Journal of Physics C:Solid State Physics, 1982(15):147−158.
[25] Zaitsev A M. Optical properties of diamond: A data handbook[M]. Springer Science and Business Media, 2013.
[26] Goss J P,Briddon P R,Hill V,et al. Identification of the structure of the 3107cm-1 H-related defect in diamond[J]. Journal of Physics:Condensed Matter, 2014, 26(14):145801. doi: 10.1088/0953-8984/26/14/145801
[27] Song Z,Su J,Zhu W,et al. Spectroscopic study of the 3107cm−1 and 3143cm−1 H-related defects in type Ib diamonds[J]. Crystals, 2022, 12:1352. doi: 10.3390/cryst12101352
[28] Eaton-Magaña S,Breeding C M. An introduction to photoluminescence spectroscopy for diamond and its applications in gemology[J]. Gems & Gemology, 2016, 52(1):2−17.
[29] Hainschwang T,Notari F,Fritsch E,et al. HPHT treatment of CO2 containing and CO2-related brown diamonds[J]. Diamond and Related Materials, 2008, 17(3):340−351. doi: 10.1016/j.diamond.2008.01.022
[30] Hainschwang T,Fritsch E,Notari F,et al. The origin of color in natural C center bearing diamonds[J]. Diamond & Related Materials, 2013, 39:27−40.
[31] Collins A T,Connor A,Ly C H,et al. High-temperature annealing of optical centers in type-I diamond[J]. Journal of Applied Physics, 2005, 97(8):83517−10. doi: 10.1063/1.1866501
[32] Yelisseyev A,Kanda H. Optical centers related to 3d transition metals in diamond[J]. New Diamond and Frontier Carbon Technology:An International Journal on New Diamond,Frontier Carbon and Related Materials[J]. New Diamond and Frontier Carbon Technology, 2007, 17(3):127−178.
[33] Nazaré M H,Neves A J. Optical studies of the 1.40-eV Ni center in diamond[J]. Physical Review B, 1991, 43(17):14196−14205. doi: 10.1103/PhysRevB.43.14196
[34] Kupriyanov I N,Gusev V A,Borzdov Y M,et al. Photoluminescence study of annealed nickel- and nitrogen-containing synthetic diamond[J]. Diamond and Related Materials, 1999, 8(7):1301−1309. doi: 10.1016/S0925-9635(99)00122-3
-




 下载:
下载: