The Pore Diffusion Coefficient of Iodide Ion in Rock Samples Using X-ray K-edge Imaging
-
摘要:
污染物质在岩层孔隙水中的扩散过程决定了污染物质在地下水与岩层之间迁移的速率,并进而影响其在环境中的迁移-转化过程。利用碘离子作为示踪剂,通过X射线成像法可以获得碘离子在岩石中随扩散距离和时间变化的扩散曲线,进而通过人工拟合获得孔隙扩散系数(Dp)以及孔隙度(φ)等重要参数,这是研究污染元素扩散行为的基础。但是在X射线成像过程中,岩石本底对X射线的吸收也会对示踪剂碘离子的成像造成干扰。本文使用X射线能谱CT利用X射线K吸收边成像法,在碘的K吸收边(33keV)两侧的能量区域(27~32keV和34~39keV)对样品进行成像,有效地减少了岩石背景干扰,获得碘离子的扩散曲线,并通过软件利用近似公式对扩散曲线进行拟合获得了灰岩中碘离子的孔隙扩散系数。在实验过程中,本文还通过改进扩散装置,对低浓度端溶液采用连续接收的方法,避免了管路中溶液死体积的影响。通过实验获得碘离子的岩石孔隙扩散系数Dp=(1.12±0.22)×10−11m2/s,并计算得到岩石孔隙度φ=0.02。此实验结果与文献中灰岩孔隙度范围(0.005~0.042)相符合。通过本次工作,验证了应用改进的扩散装置和X射线K吸收边成像方法测量碘离子在岩石中的孔隙扩散系数的可行性。
Abstract:BACKGROUND The diffusion process is important for environmental studies and pollution monitoring. When contaminants are released into groundwater in the natural environment, they can diffuse into pore water in the rock matrix driven by the concentration gradients. When the contamination source is removed after remediation, the contaminated porewater can be a long-term contamination source to the groundwater in fractures via back diffusion. The diffusion coefficients are the key parameters for the studies of the diffusion-reaction process of contaminants in the environment, especially in natural porous rocks. The diffusion method, which measures the steady-state diffusion flux of non-sorbing tracers, such as I− and HTO, has been widely used for the determination of diffusion coefficients. X-ray radiography techniques, which are non-destructive and can monitor temporal changes of tracers in the porous rock medium, have been developed to obtain the pore diffusion coefficients in rock samples by fitting a solution of Fick's second law of diffusion to profiles of Δμ (the change in the X-ray attenuation coefficient). The value of Δμ is a function of the mass of a tracer along the X-ray path. However, the X-ray attenuation by the rock matrix interferes with the determination of tracer signals, due to the low concentration of the tracer in the porewaters. The method of X-ray K-Edge imaging using a photon counting detector (PCD), which takes images from the two energy bins on both sides of the theoretical K-edge position of the tracer element and obtains the image subtraction, can be used for the determination of diffusion coefficients to reduce the interferences from the rock background. This work focuses on the diffusion process of iodide ion in limestone samples. X-ray K-edge imaging with energy-resolved computed tomography (CT) has been employed to image the iodide ion tracer in limestone samples. The energy range is on both sides of the iodine K-edge (33keV) for X-ray K-edge imaging.
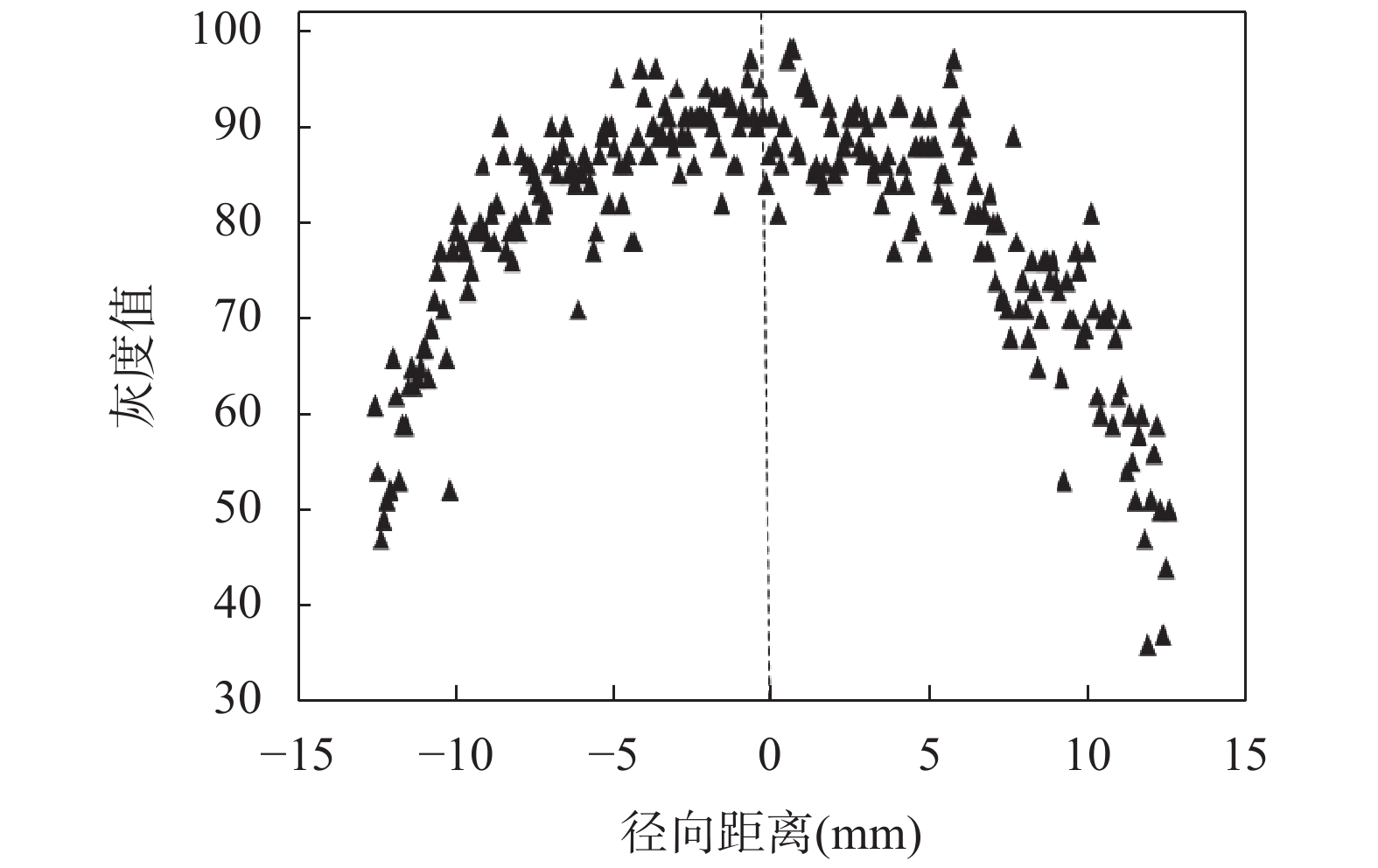
RESULTS Modification of diffusion device. In this work, the diffusion device were modified to continuously collect the low-concentration solution (Fig.1), which prevented the interference from the dead volume. The rock samples were placed in sample tubes and inserted between the upper and lower protrusions of the sample cell. A high concentration potassium iodide (KI) solution and a blank solution (deionied water) were pumped into the lower and upper surface of the rock samples, respectively. Driven by a concentration gradient, iodide ion diffused from the lower surface to the upper surface and was washed out by the blank solution. After a certain period of time, sub-samples of blank solution were collected for measuring the iodide ion concentration. X-ray K-edge imaging. The method of X-ray K-edge imaging was applied to obtain the iodide ion tracer diffusion curve in the limestone sample using a spectral computed tomography. The X-ray energy thresholds were set at 27-32keV and 34-39keV (Table 1), which were on both sides of the iodine K-edge position (33keV). Diffusion experiments. The limestone samples, which were collected from Changxing, Zhejiang, China, were cut as cylindrical samples (2.5cm diameter, 1-2cm length). Subsample A, B and C were the blank sample (no iodide tracer), the sample for diffusion experiment, and the tracer-saturated sample (saturated with iodide tracer), respectively. Sample B was mounted in a diffusion cell and then placed on the diffusion experiment device (Fig.1). The iodide solution(KI, 1mol/L)was then circulated in the tracer reservoir and the low concentration solution deionied water)was collected. Diffusion coefficient measurements. The limestone sample was taken out of the diffusion cell for X-ray K-edge imaging in an X-ray spectral CT from the second day after the diffusion experiment was started. The original images were converted into 8-bit grayscale images using ImageJ software, where the grayscale values were correlated with the normalized iodide tracer concentrations (Ct /C0). To minimize the shape effect of cylindrical samples, the average greyscale values, which were selected from the area of ±5mm to the center of radial direction, were used for calculating the iodide ion pore diffusion coefficient. The normalized concentration (Ct /C0) is a function of diffusion distance (x) and diffusion time (t), which has an analytical solution as $\dfrac{C_t}{c_0}=\operatorname{erfc}\left(\dfrac{x}{2 \sqrt{D_{\mathrm{p}} t}}\right)$, where erfc is the complimentary error function and Dp is the pore diffusion coefficient. The diffusion curve data (Fig.3) were fitted with an approximate formula of complementary error function (Eq.3) using SigmaPlot 14. The results show that the pore diffusion coefficient, Dp, is (1.12±0.22)×10−11 m2s−1. Before starting the X-ray radiography experiment, a through diffusion experiment was conducted on the limestone sample (24.74mm diameter, 14.73mm length). The iodide ion concentration was determined using ICP-MS and the steady-state iodide flux was used to calculate the effective diffusion coefficient, De, based on Fick’s first low of diffusion. The effective diffusion coefficient Dp for limestone samples was (2.2±0.7)×10−13m2s−1 and porosity φ was 0.02 calculated by De=φ·Dp.
DISSCUSION In previous studies on diffusion coefficient measurements in rocks using X-ray radiography techniques, the X-ray source generates a continuous energy spectrum of X-rays, and the grayscale of the resulting image reflects the average attenuation coefficient of X-rays within the energy range passing through all rock matrices. The background of the rock, which is larger in mass than the tracer (iodide ion), can cause significant interference. In this work, the X-ray K-edge imaging method has been used. Within this selected energy ranges on both sides of the iodine K-edge, there is a noticeable difference in the attenuation coefficients between the rock background and iodide ion. The tracer iodide ion can be effectively separated from the rock background, which improves the signal-to-noise ratio. In previous studies, fitting of the complementary error function has often been done by manual parameter adjustment, which can introduce artificial uncertainties. In this study, a modified diffusion device is used, and an approximate formula of the complementary error function is applied for data fitting. This approach minimizes systematic errors from both the experimental setup and data processing. The iodide ion is easily oxidized in the environment by an oxidant, such as oxygen. This oxidation can lead to changes in the diffusion coefficient of iodide ion. In this work, sodium thiosulfate is added to the iodide tracer solution to prevent the oxidation of the iodide ion. The relative iodide ion concentration profiles of sample B (Fig.3) show that the iodide concentration is decreased with increasing diffusion distance and the diffusion curve become flat with increasing experimental time. Dp for the first sampling deviates from the average Dp values. This can be attributed to the fact that during the initial stages of the diffusion experiment, the iodide ion may not have formed an ideal diffusion curve in the limestone samples. As a result, the diffusion coefficient obtained from diffusion curve fitting deviates significantly. The De value obtained from this work agrees with previous studies, in which the De value ranges from 5.3×10−14m2·s−1 to 5.6×10−13m2·s−1. Correspondingly, the porosity of the limestone obtained in this study is 0.02, which is consistent with the reported porosity range of limestone in the literature (0.005-0.042). These results validate that this method will be feasible for determining the pore diffusion coefficients and porosities in rock samples. The method development of X-ray K-edge imaging using an X-ray spectral CT provides a method to obtain the pore diffusion coefficients and reduce the interference from the rock matrix. Since the concentration changes when the experiment is paused for X-ray CT imaging can introduce disturbances to the entire diffusion process, the experimental results can be obviously affected. In future work, diffusion devices might be modified to be placed inside X-ray CT. Therefore, X-ray K-edge imaging of samples can be carried out without interrupting the diffusion process, avoiding any disruptions that may affect the results.
-

-
表 1 能谱显微CT系统成像参数
Table 1. Parameters for X-ray spectral microcomputed tomography (CT) system.
系统参数 测量条件 曝光条件 90kVp,17.28mAs 能量阈值设计 27~32keV和34~39keV 光机到探测器距离 360mm 光机到物体距离 340mm 图像像素尺寸 94.2μm/pixel 表 2 X射线K吸收边成像样品孔隙扩散系数的拟合结果
Table 2. The results for the X-ray K-edge imaging data fitting.
采样顺序编号 扩散实验时间
(h)孔隙扩散系数
Dp(×10−11m2/s)1 42 3.08 2 66 1.19 3 90 1.31 4 119 0.80 5 138 1.20 -
[1] 曾远,罗立强. 土壤中特异性微生物与重金属相互作用机制与应用研究进展[J]. 岩矿测试, 2017, 36(3): 209−221. doi: 10.15898/j.cnki.11-2131/td.201701170009
Zeng Y,Luo L Q. Research progress on the application and interaction mechanism between specific microorganisms and heavy metals in soil[J]. Rock and Mineral Analysis, 2017, 36(3): 209−221. doi: 10.15898/j.cnki.11-2131/td.201701170009
[2] 董志高,付婷婷,李磊,等. 某简易污泥填埋场污染物调查与扩散迁移研究[J]. 科学技术与工程, 2014, 14(32): 1671−1815.
Dong Z G,Fu T T,Li L,et al. Study on investigation,diffusion and migration of pollutants in a simple sewage sludge landfill[J]. Science Technology and Engineering, 2014, 14(32): 1671−1815.
[3] Luraschi P,Gimmi T,van Loon L R,et al. Evolution of HTO and 36Cl− diffusion through a reacting cement-clay interface (OPC paste-Na montmorillonite) over a time of six years[J]. Applied Geochemistry, 2020, 119: 104581. doi: 10.1016/j.apgeochem.2020.104581
[4] Courtin-Nomade A,Waltzing T,Evrard C,et al. Arsenic and lead mobility:From tailing materials to the aqueous compartment[J]. Applied Geochemistry, 2015, 64: 10−21.
[5] Cutruneo C M N L,Oliveira M L S,Ward C R,et al. A mineralogical and geochemical study of three Brazilian coal cleaning rejects:Demonstration of electron beam applications[J]. International Journal of Coal Geology, 2014, 130: 33−52. doi: 10.1016/j.coal.2014.05.009
[6] Liao X,Li Y,Yan X. Removal of heavy metals and arsenic from a co-contaminated soil by sieving combined with washing process[J]. Journal of Environmental Sciences, 2016, 41: 202−210. doi: 10.1016/j.jes.2015.06.017
[7] Halloran L J S,Vakili F,Wanner P,et al. Sorption- and diffusion-induced isotopic fractionation in chloroethenes[J]. Science of the Total Environment, 2021, 788: 147826. doi: 10.1016/j.scitotenv.2021.147826
[8] Parker B L,Cherry J A,Chapman S W. Discrete fracture network approach for studying contamination in fractured rock[J]. AQUA mundi, 2012, 3: 101−116.
[9] Zhao J,Al T,Chapman S W,et al. Determination of hexavalent chromium concentrations in matrix porewater from a contaminated aquifer in fractured sedimentary bedrock[J]. Chemical Geology, 2015, 419: 142−148. doi: 10.1016/j.chemgeo.2015.10.034
[10] Muniruzzaman M,Rolle M. Impact of diffuse layer processes on contaminant forward and back diffusion in heterogeneous sandy-clayey domains[J]. Journal of Contaminant Hydrology, 2021, 237: 103754. doi: 10.1016/j.jconhyd.2020.103754
[11] Chapman S,Parker B,Al T,et al. Field,laboratory and modeling evidence for strong attenuation of a Cr(Ⅵ) plume in a mudstone aquifer due to matrix diffusion and reaction processes[J]. Soil System, 2021(Ⅵ): 408−413.
[12] Cavé L,Al T,Xiang Y,et al. A technique for estimating one-dimensional diffusion coefficients in low-permeability sedimentary rock using X-ray radiography:Comparison with through-diffusion measurements[J]. Journal of Contaminant Hydrology, 2009, 103(1-2): 1−12. doi: 10.1016/j.jconhyd.2008.08.001
[13] Xiang Y,Al T. Effect of confining pressure on diffusion coefficients in low-permeability Ordovician sedimentary rocks from the Michigan Basin,Southwestern Ontario[J]. Journal of Contaminant Hydrology, 2016, 195: 2003.
[14] Nunn J A,Xiang Y,Al T A. Investigation of partial water saturation effects on diffusion in shale[J]. Applied Geochemistry, 2018, 97: 93−101. doi: 10.1016/j.apgeochem.2018.08.004
[15] Loomer D B,Scott L,Al T A,et al. Diffusion-reaction studies in low permeability shale using X-ray radiography with cesium[J]. Applied Geochemistry, 2013, 39: 49−58. doi: 10.1016/j.apgeochem.2013.09.019
[16] van Loon L R,Soler J M,Jakob A,et al. Effect of confining pressure on the diffusion of HTO,36Cl− and 125I− in a layered argillaceous rock (Opalinus Clay):Diffusion perpendicular to the fabric[J]. Applied Geochemistry, 2003, 18(10): 1653−1662. doi: 10.1016/S0883-2927(03)00047-7
[17] van Loon L R,Soler J M,Bradbury M H. Diffusion of HTO,36Cl- and 125I- in Opalinus Clay samples from Mont Terri:Effect of confining pressure[J]. Journal of Contaminant Hydrology, 2003, 61(1-4): 73−83. doi: 10.1016/S0169-7722(02)00114-6
[18] Jaquenoud M, Elam W T, Grundl T, et al. In-situ X-ray fluorescence to investigate iodide diffusion in Opalinus clay: Demonstration of a novel experimental approach[J]. Chemosphere, 2021, 269.
[19] 陈维堃,腾格尔,张春贺,等. 页岩纳米有机孔结构表征技术研究进展[J]. 岩矿测试, 2022, 41(6): 906−919.
Chen W K,Teng G E,Zhang C H,et al. A review of research progress on characterization technology of nano organic pore structure in shale[J]. Rock and Mineral Analysis, 2022, 41(6): 906−919.
[20] Wu Y,Wang D,Wang L,et al. An analysis of the meso-structural damage evolution of coal using X-ray CT and a gray-scale level co-occurrence matrix method[J]. International Journal of Rock Mechanics and Mining Sciences, 2022, 152: 105062. doi: 10.1016/j.ijrmms.2022.105062
[21] Xiang Y,Al T,Scott L,et al. Diffusive anisotropy in low-permeability Ordovician sedimentary rocks from the Michigan Basin in Southwest Ontario[J]. Journal of Contaminant Hydrology, 2013, 155: 31−45. doi: 10.1016/j.jconhyd.2013.09.002
[22] Zhang Z,Zhang X,Hu J,et al. An optimized K-edge signal extraction method for K-edge decomposition imaging using a photon counting detector[J]. Frontiers in Physics, 2021, 8: 1−12.
[23] 李非,蔡婧婧. 双能量CT成像技术及其标准研究[J]. 中国医疗器械信息, 2022, 22(9): 10−15. doi: 10.3969/j.issn.1006-6586.2022.09.004
Li F,Cai Q Q. Study on dual-energy CT imaging technology and its standard[J]. China Medical Device Information, 2022, 22(9): 10−15. doi: 10.3969/j.issn.1006-6586.2022.09.004
[24] Iovea M,Oaie G,Ricman C,et al. Dual-energy X-ray computer axial tomography and digital radiography investigation of cores and other objects of geological interest[J]. Engineering Geology, 2009, 103(3-4): 119−126. doi: 10.1016/j.enggeo.2008.06.018
[25] Gupta A,Kikano E G,Bera K,et al. Dual energy imaging in cardiothoracic pathologies:A primer for radiologists and clinicians[J]. European Journal of Radiology Open, 2021, 8: 100324. doi: 10.1016/j.ejro.2021.100324
[26] Zhao T,Li L,Chen Z. Dynamic material decomposition method for MeV dual-energy X-ray CT[J]. Applied Radiation and Isotopes, 2018, 140: 55−62. doi: 10.1016/j.apradiso.2018.06.009
[27] Zhou S,Zhu L,You T,et al. In vivo quantification of bone mineral density of lumbar vertebrae using fast kVp switching dual-energy CT:Correlation with quantitative computed tomography[J]. Quantitative Imaging in Medicine and Surgery, 2021, 11(1): 341−350. doi: 10.21037/qims-20-367
[28] Flohr T,Petersilka M,Henning A,et al. Photon-counting CT review[J]. Physica Medica, 2020, 79: 126−136. doi: 10.1016/j.ejmp.2020.10.030
[29] Wigger C,van Loon L R. Effect of the pore water composition on the diffusive anion transport in argillaceous,low permeability sedimentary rocks[J]. Journal of Contaminant Hydrology, 2018, 213: 40−48. doi: 10.1016/j.jconhyd.2018.05.001
[30] 赵鸿,杜安道,李超,等. 浙江长兴“金钉子”灰岩Re-Os富集机制研究[J]. 地质学报, 2015, 89(10): 1783−1791.
Zhao H,Du A D,Li C,et al. Enrichment mechanism of Re-Os in limestone from Changxing Permian—Triassic boundary in Zhejiang[J]. Acta Geologica Sinica, 2015, 89(10): 1783−1791.
[31] Bercu G. New refinements for the error function with applications in diffusion theory[J]. Symmetry, 2020, 12(12): 1−13.
[32] Wang B T,Lee C P,Wu M C,et al. Novel method for analyzing transport parameters in through-diffusion tests[J]. Journal of Environmental Radioactivity, 2019, 196: 125−132.
[33] Vedder J D. Simple approximations for the error function and its inverse[J]. American Journal of Physics, 1987, 55(8): 762−763. doi: 10.1119/1.15018
[34] 郭俊克,郭苏凯. 误差函数的有限形式式近似式及其应用[J]. 太原工业大学学报, 1988, 19(3): 17−24.
Guo J K,Guo S K. Finite approximate formula of error function and its applications[J]. Journal of Taiyuan University of Technology, 1988, 19(3): 17−24.
[35] 田锦州,徐乃忠,李凤明. 误差函数erf(x)近似计算及其在开采沉陷预计中的应用[J]. 煤矿开采, 2009, 14(2): 33−35.
Tian J Z,Xu N Z,Li F M. Proximate calculation of error function erf (x) and its application in mining subsidence prediction[J]. Coal Mining Technology, 2009, 14(2): 33−35.
[36] MacKeown H,von Gunten U,Criquet J. Iodide sources in the aquatic environment and its fate during oxidative water treatment—A critical review[J]. Water Research, 2022, 217: 118417. doi: 10.1016/j.watres.2022.118417
[37] Ye T,Zhang T Y,Tian F X,et al. The fate and transformation of iodine species in UV irradiation and UV-based advanced oxidation processes[J]. Water Research, 2021, 206: 117755. doi: 10.1016/j.watres.2021.117755
[38] Neil C W,Telfeyan K,Sauer K B,et al. Iodine effective diffusion coefficients through volcanic rock:Influence of iodine speciation and rock geochemistry[J]. Journal of Contaminant Hydrology, 2020, 235: 103714. doi: 10.1016/j.jconhyd.2020.103714
-




 下载:
下载: