High Precision Strontium Isotope Measurement of Rock Standard Materials by Multi-dynamic TIMS
-
摘要:
铷-锶(Rb-Sr)同位素体系的放射性同位素衰变规律可以用于分析成矿物质来源和确定成矿时代,对于理解矿床的形成过程、确定矿床的成因以及对进一步找矿都具有十分重要的作用。多接收表面热电离质谱(MC-TIMS)是当前地质样品高精度锶同位素组成分析的首选技术,但法拉第杯之间的杯系数差异严重制约了仪器测量的精确度和准确度。本文采用动态多接收校正方法开展了TIMS的高精度锶同位素分析,通过蒙特卡洛模拟和实际测量论证了这一方法在提高锶同位素分析精确度和准确度方面的有效性。结果表明,采用动态多接收校正方法能够有效地消除杯系数的影响,不仅能确保测量的准确度,还能提高2~3倍的测量精确度,实现优于8ppm的仪器长期测试精度。地质样品的化学前处理采用AG 50W-X8树脂和Sr特效树脂两柱可以实现分离。淋洗曲线实验表明该分离流程具有很好的普适性,全流程空白小于150pg,锶回收率≥95%。采用本方法对13种具有不同岩性和锶含量的国家标准物质进行了高精度锶同位素分析测定,其中9种为首次报道,丰富了该系列标样的锶同位素数据库,为更广泛的地质应用提供有力支撑。
-
关键词:
- 动态多接收 /
- 多接收表面热电离质谱 /
- 杯系数 /
- 锶同位素 /
- 国家标准物质
Abstract:BACKGROUND Strontium isotopes are a powerful geochemical indicator for tracing the sources and ages of ore-forming materials. TIMS is internationally recognized as the “gold standard” for determining Sr isotopic compositions, however, its analysis accuracy is severely limited by the attenuation of Faraday cup efficiency. How to effectively eliminate the influence of Faraday cup efficiency change is the key to improving the accuracy of Sr isotopes measured by TIMS. In addition, matrix reference materials are crucial for validating measurements on geological samples. Therefore, it is necessary to calibrate the Sr isotope composition of new geological reference materials to replace the unavailable standards (e.g., USGS).
OBJECTIVES To develop a high-precision Sr isotope analysis method using multi-dynamic TIMS and accurately calibrate a set of GBW reference materials with varying sample matrices.
METHODS Samples were completely digested by the high-pressure bomb method. Complete separation of Sr from sample matrices was accomplished through a two-stage column separation method consisting of ion exchange resin (AG 50X-12) and extraction resin (Sr spec). Sr isotopes were measured using a multi-collector TIMS and collected in a three-lines cup configuration. The internal normalization method was used to correct for instrument mass bias, and the multi-dynamic collection method was employed to mitigate the effects of Faraday cup efficiency drift.
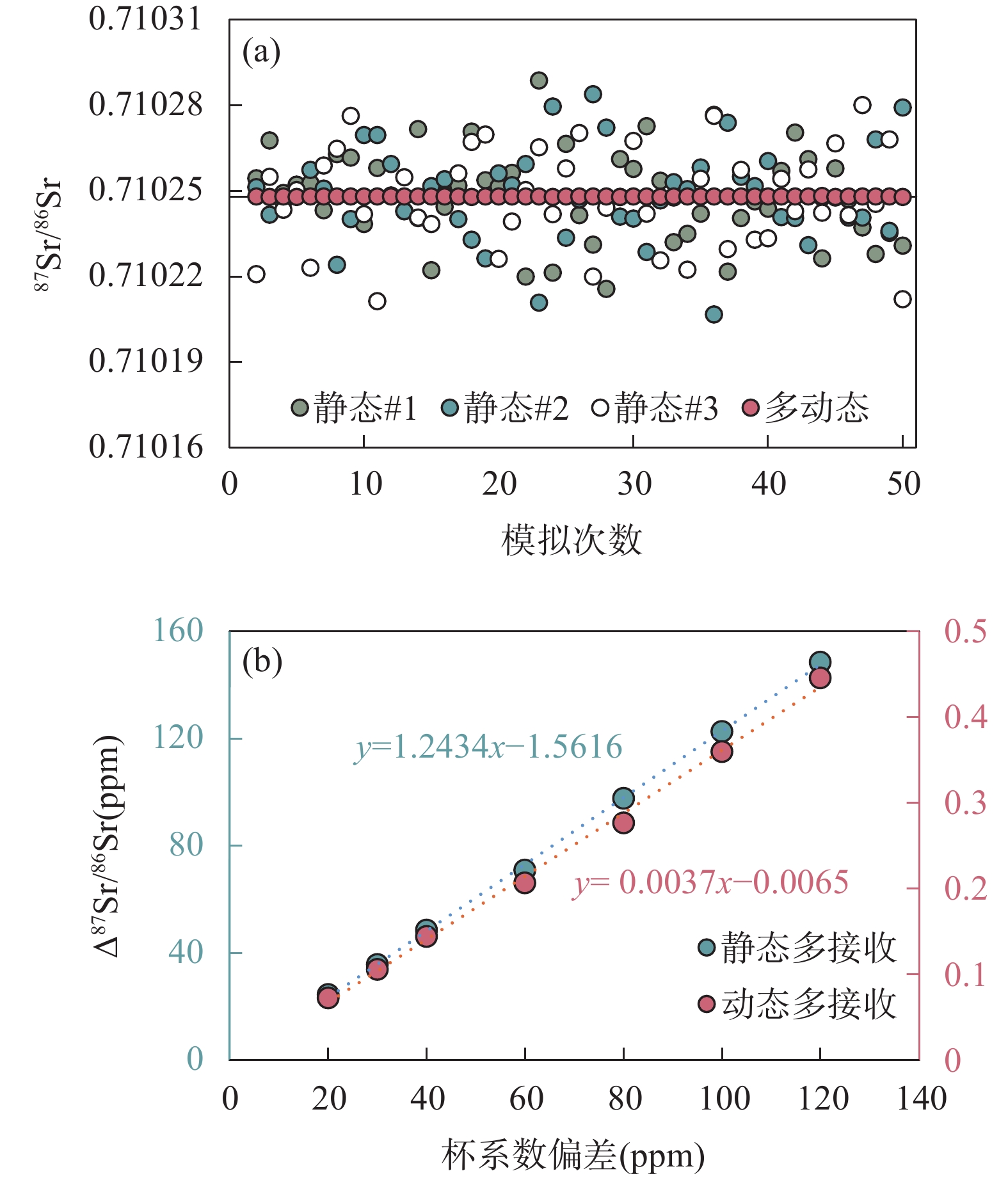
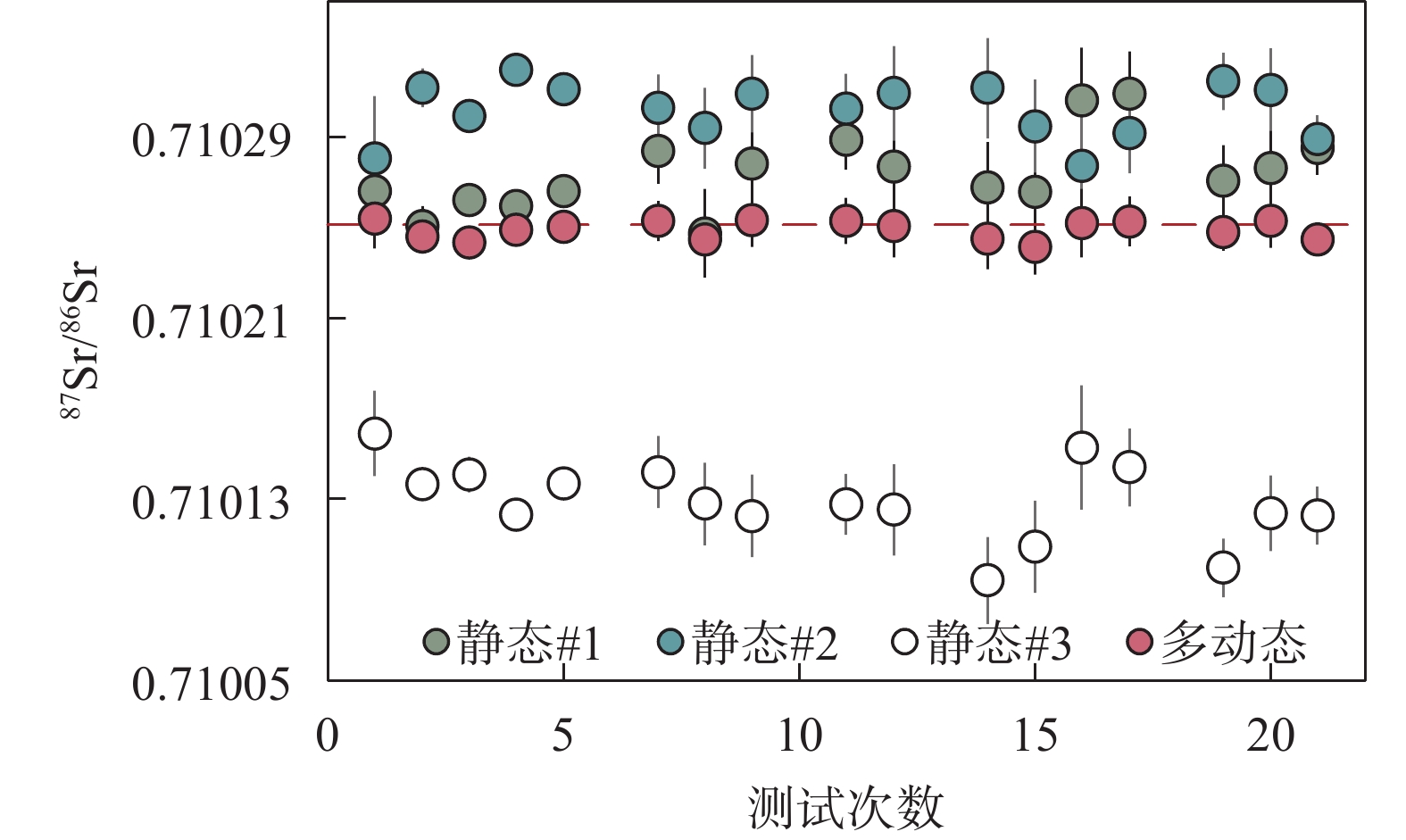
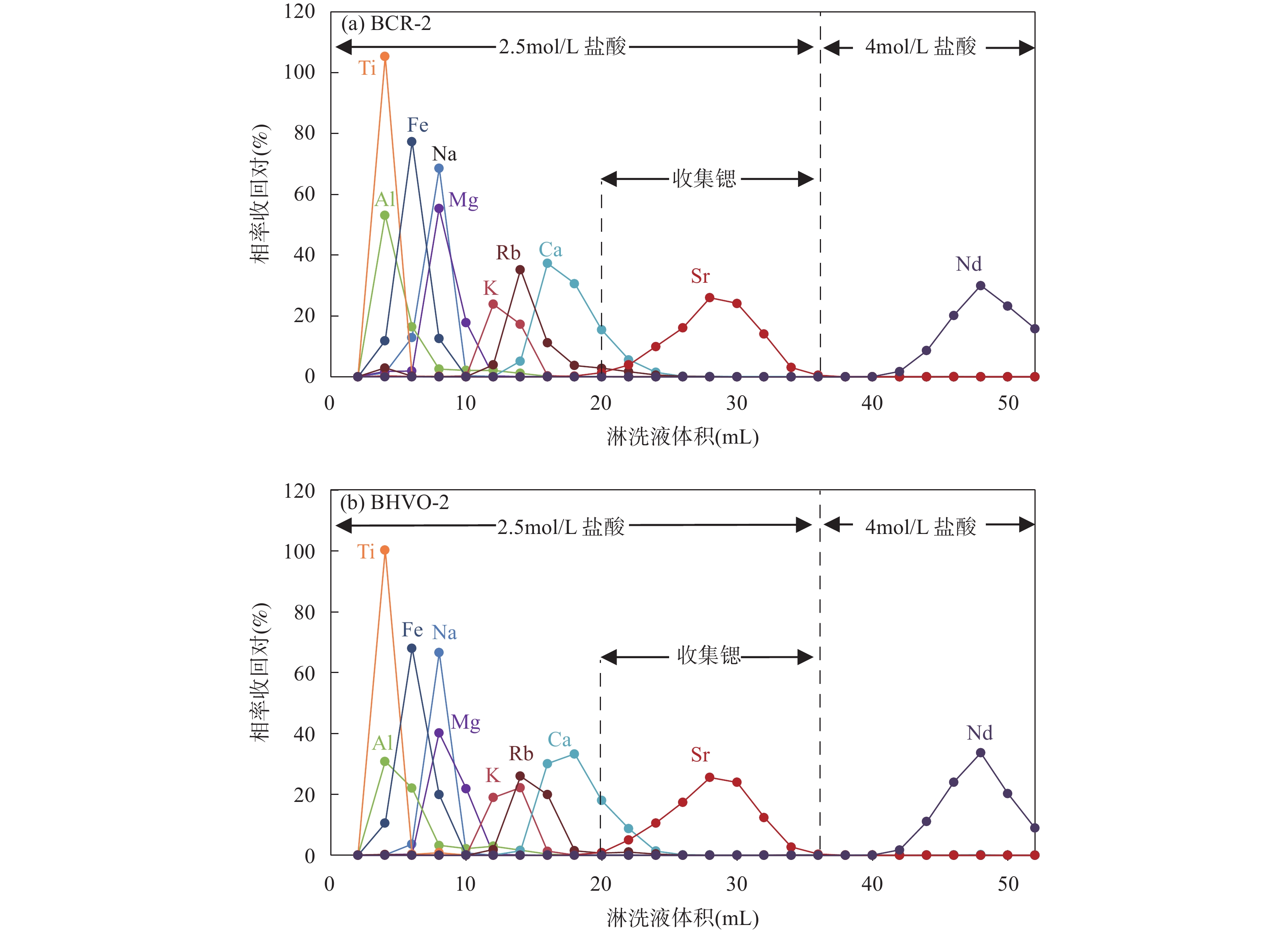
RESULTS (1) Significant Faraday cup deterioration (up to 160μg/g on C cup) was observed during an 8 months Sr isotope analytical session. Nevertheless, the results from the Monte Carlo simulation indicate that the multi-dynamic collection method can eliminate 99.6% of the cup coefficient effect. Moreover, long-term testing of NBS987 shows that employing the multi-dynamic collection method results in an instrumental precision of 8μg/g, which is 2-3 times more accurate than traditional static collection methods. Overall, results from both theoretical predictions and practical testing confirmed that the multi-dynamic collection method can effectively eliminate the effects of the cup effect drift. (2) The Sr recovery of the leaching experiments for BCR-2 and BHVO-2 with AG 50W-X8 resin column were 99.16% and 98.91%, respectively. The total Sr recovery of the two-stage columns was as high as 95%, thus preventing any potential Sr isotope fractionation resulting from Sr losses during the column separation process. Furthermore, the total blank throughout the entire procedure was no higher than 150pg for Sr, which was negligible compared to the large sample size. (3) High precision Sr isotope compositions were determined for 13 geological reference samples with various sample matrices, resulting in 87Sr/86Sr ratio measurements ranging from 0.704078 to 0.807402. Among these results, GBW07104, GBW07105, GBW07106, and GBW07108 were found to be consistent with previously reported values within the uncertainties and the other nine reference materials were reported herein for the first time.
CONCLUTIONS The cup effect can significantly impact the Sr isotope measured by MC-TIMS, but it can be effectively mitigated by using a multi-dynamic collection method. Furthermore, independent test results demonstrate the uniformity of the Sr isotope composition in these GBW standards, rendering them suitable for both quality control and interlaboratory comparison purposes.
-
Key words:
- multi-dynamic collection /
- MC-TIMS /
- cup efficiency /
- strontium isotope /
- GBW reference materials
-

-
表 1 锶同位素的两柱化学纯化流程
Table 1. Two-stage chemical separation of strontium isotope.
离子交换树脂 淋洗步骤 淋洗液类型 淋洗液体积
(mL)第一柱
(AG 50W-X8)洗柱 6mol/L盐酸 30 洗柱 高纯水 10 平衡柱 2.5mol/L盐酸 5 上样 2.5mol/L盐酸 1 淋洗样品 2.5mol/L盐酸 1 淋洗杂质元素 2.5mol/L盐酸 4×4 收集锶 2.5mol/L盐酸 15 第二柱
(Sr特效树脂)洗柱 3mol/L硝酸 1×3 洗柱 高纯水 1×3 平衡柱 3mol/L硝酸 1 上样 3mol/L硝酸 0.2 淋洗样品 3mol/L硝酸 0.4 淋洗杂质元素 3mol/L硝酸 1×3 收集锶 高纯水 2 表 2 锶同位素分析采用的动态多接收杯结构
Table 2. Multi-dynamic cup configuration used for strontium isotope analysis in this study.
法拉第杯 L3 L2 L1 C H1 H2 H3 聚焦电压
(V)色散电压
(V)子杯结构1 − − 84Sr 85Rb 86Sr 87Sr 88Sr −2 8 子杯结构2 − 84Sr 85Rb 86Sr 87Sr 88Sr − 0 0 子杯结构3 84Sr 85Rb 86Sr 87Sr 88Sr − − 2 −8 注:表中“−”表示对应杯中不接收任何同位素信息。 表 3 13种国家标准物质的锶同位素组成
Table 3. Strontium isotope composition of 13 Chinese geological reference materials.
标准物质编号 岩性 Sr含量
(μg/g)87Sr/86Sr 不确定度* 测量仪器 文献来源 GBW07104
(GSR-2)安山岩 790 0.704917 0.000011 − − 0.704918 0.000011 − − 0.704919 0.000014 − − 0.704918 0.000002 TIMS 本文 0.704929 0.000012 TIMS Guo等(2023)[15] 0.704931 0.000032 MC-ICP-MS Chen等(2022)[38] 0.704914 0.000030 TIMS Yang等(2020)[4] GBW07105
(GSR-3)玄武岩 1100 0.704079 0.000013 − − 0.704080 0.000011 − − 0.704076 0.000013 − − 0.704076 0.000012 − − 0.704078 0.000005 TIMS 本文 0.704076 0.000036 TIMS Fourny等(2016)[39] 0.704093 0.000010 TIMS Guo等(2023) [15] 0.704081 0.000016 MC-ICP-MS Chen等(2022)[38] 0.704090 0.000016 MC-ICP-MS Wu等(2021)[40] GBW07106
(GSR-4)石英砂岩 58 0.719944 0.000012 − − 0.719948 0.000015 − − 0.719909 0.000011 − − 0.719898 0.000030 − − 0.719925 0.000050 TIMS 本文 0.719936 0.000052 MC-ICP-MS Chen等(2022)[38] GBW07107
(GSR-5)页岩 90 0.807405 0.000011 − − 0.807413 0.000016 − − 0.807390 0.000014 − − 0.807399 0.000016 − − 0.807402 0.000019 TIMS 本文 GBW07108
(GSR-6)泥质灰岩 913 0.708383 0.000012 − − 0.708407 0.000013 − − 0.708382 0.000011 − − 0.708399 0.000012 − − 0.708393 0.000024 TIMS 本文 0.708406 0.000060 MC-ICP-MS Chen等(2022)[38] GBW07112
(GSR-10)辉长岩 612 0.704383 0.000011 − − 0.704382 0.000012 − − 0.704371 0.000010 − − 0.704379 0.000014 TIMS 本文 GBW07114
(GSR-12)白云岩 27 0.708334 0.000013 − − 0.708327 0.000018 − − 0.708348 0.000051 − − 0.708336 0.000022 TIMS 本文 GBW07120
(GSR-13)石灰岩 107 0.709394 0.000013 − − 0.709430 0.000012 − − 0.709416 0.000012 − − 0.709414 0.000036 TIMS 本文 GBW07121
(GSR-14)花岗片麻岩 690 0.709400 0.000012 − − 0.709401 0.000013 − − 0.709396 0.000012 − − 0.709399 0.000005 TIMS 本文 GBW07725
(GSR-16)含铀砂岩 252 0.726437 0.000012 − − 0.726436 0.000013 − − 0.726437 0.000001 TIMS 本文 GBW07726
(GSR-17)二辉斜长麻粒岩 818 0.704278 0.000016 − − 0.704297 0.000011 − − 0.704294 0.000013 − − 0.704289 0.000021 TIMS 本文 GBW07727
(GSR-18)峨眉山玄武岩 271 0.705919 0.000014 − − 0.705922 0.000013 − − 0.705920 0.000011 − − 0.705921 0.000003 TIMS 本文 GBW07728
(GSR-19)辉石橄榄岩 38 0.708531 0.000012 − − 0.708519 0.000014 − − 0.708529 0.000014 − − 0.708509 0.000017 − − 0.708522 0.000020 TIMS 本文 注:表中“-”代表本实验TIMS的地质标样的单次测试结果;“*”示意表中不确定度的表示方法有两种情况,单次测试结果的不确定度为2SE(带“-”符号),多次测试平均结果的不确定度为2SD(粗体),文献报道值的不确定度也为2SD。
-
[1] 韦刚健, 刘颖, 涂湘林, 等. 利用选择性特效树脂富集分离岩石样品中的锶钐和钕[J]. 岩矿测试, 2004, 23(1): 11−14. doi: 10.3969/j.issn.0254-5357.2004.01.003
Wei G J, Liu Y, Tu X L, et al. Separation of Sr, Sm and Nd in mineral and rock samples using selective specific resins[J]. Rock and Mineral Analysis, 2004, 23(1): 11−14. doi: 10.3969/j.issn.0254-5357.2004.01.003
[2] 赵葵东, 蒋少涌. 金属矿床的同位素直接定年方法[J]. 地学前缘, 2004, 11(2): 425−434. doi: 10.3321/j.issn:1005-2321.2004.02.012
Zhao K D, Jiang S Y. Direct isotope dating for metallic ore deposits[J]. Earth Science Frontiers, 2004, 11(2): 425−434. doi: 10.3321/j.issn:1005-2321.2004.02.012
[3] Li C F, Chu Z Y, Wang X C, et al. Sr isotope analysis of picogram-level samples by thermal ionization mass spectrometry using a highly sensitive silicotungstic acid emitter[J]. Analytical Chemistry, 2019, 91(11): 7288−7294. doi: 10.1021/acs.analchem.9b00958
[4] Yang Y H, Yang M, Jochum K P, et al. High-precision Sr-Nd-Hf-Pb isotopic composition of Chinese geological standard glass reference materials CGSG-1, CGSG-2, CGSG-4 and CGSG-5 by MC-ICP-MS and TIMS[J]. Geostandards and Geoanalytical Research, 2020, 44(3): 567−579. doi: 10.1111/ggr.12322
[5] Luu T H, Gutiérrez P, Inglis E C, et al. High-precision Sr and Nd isotope measurements using a dynamic zoom lens-equipped thermal ionisation mass spectrometer[J]. Chemical Geology, 2022, 611: 121078. doi: 10.1016/j.chemgeo.2022.121078
[6] Di Y, Krestianinov E, Zink S, et al. High-precision multidynamic Sr isotope analysis using thermal ionization mass spectrometer (TIMS) with correction of fractionation drift[J]. Chemical Geology, 2021, 582: 120411. doi: 10.1016/j.chemgeo.2021.120411
[7] Thirlwall M F, Anczkiewicz R. Multidynamic isotope ratio analysis using MC-ICP-MS and the causes of secular drift in Hf, Nd and Pb isotope ratios[J]. International Journal of Mass Spectrometry, 2004, 235(1): 59−81. doi: 10.1016/j.ijms.2004.04.002
[8] Makishima A, Nakamura E. Calibration of Faraday cup efficiency in a multicollector mass spectrometer[J]. Chemical Geology, 1991, 94(2): 105−110. doi: 10.1016/0168-9622(91)90003-F
[9] Miyazaki T, Vaglarov B S, Kimura J I. Determination of relative Faraday cup efficiency factor using exponential law mass fractionation model for multiple collector thermal ionization mass spectrometry[J]. Geochemical Journal, 2016, 50(5): 445−447. doi: 10.2343/geochemj.2.0439
[10] Krabbenhöft A, Fietzke J, Eisenhauer A, et al. Determination of radiogenic and stable strontium isotope ratios (87Sr/86Sr; δ88/86Sr) by thermal ionization mass spectrometry applying an 87Sr/84Sr double spike[J]. Journal of Analytical Atomic Spectrometry, 2009, 24(9): 1267. doi: 10.1039/b906292k
[11] Di Y, Li Z, Amelin Y. Monitoring and quantitative evaluation of Faraday cup deterioration in a thermal ionization mass spectrometer using multidynamic analyses of laboratory standards[J]. Journal of Analytical Atomic Spectrometry, 2021, 36(7): 1489−1502. doi: 10.1039/D1JA00028D
[12] Yobregat E, Fitoussi C, Bourdon B. A new method for TIMS high precision analysis of Ba and Sr isotopes for cosmochemical studies[J]. Journal of Analytical Atomic Spectrometry, 2017, 32(7): 1388−1399. doi: 10.1039/C7JA00012J
[13] Feng L, Zhou L, Yang L, et al. Optimization of double spike technique using peak jump collection by Monte Carlo method: An example for the determination of Ca isotope ratios[J]. Journal of Analytical Atomic Spectrometry, 2015, 30(12): 2403−2411. doi: 10.1039/C5JA00203F
[14] Yang L, Tong S, Zhou L, et al. A critical review on isotopic fractionation correction methods for accurate isotope amount ratio measurements by MC-ICP-MS[J]. Journal of Analytical Atomic Spectrometry, 2018, 33(11): 1849−1861. doi: 10.1039/C8JA00210J
[15] Guo K, Yu J, Fan D, et al. Precise determination of Sr and Nd isotopic compositions of Chinese standard reference samples GSR-1, GSR-2, GSR-3 and GBW07315 by TIMS[J]. Geosystems and Geoenvironment, 2023, 2(4): 100206. doi: 10.1016/j.geogeo.2023.100206
[16] 唐索寒, 李津, 潘辰旭, 等. 岩石铷-锶和钐-钕同位素标准物质的研制[J]. 岩矿测试, 2021, 40(2): 285−295. doi: 10.15898/j.cnki.11-2131/td.202011110140
Tang S H, Li J, Pan C X, et al. Preparation of the reference materials for Rb-Sr and Sm-Nd isotope analysis[J]. Rock and Mineral Analysis, 2021, 40(2): 285−295. doi: 10.15898/j.cnki.11-2131/td.202011110140
[17] Birck J L. Precision K-Rb-Sr isotopic analysis: Application to Rb-Sr chronology[J]. Chemical Geology, 1986, 56(1-2): 73−83. doi: 10.1016/0009-2541(86)90111-7
[18] Charlier B L A, Ginibre C, Morgan D, et al. Methods for the microsampling and high-precision analysis of strontium and rubidium isotopes at single crystal scale for petrological and geochronological applications[J]. Chemical Geology, 2006, 232(3-4): 114−133. doi: 10.1016/j.chemgeo.2006.02.015
[19] Meynadier L, Gorge C, Birck J L, et al. Automated separation of Sr from natural water samples or carbonate rocks by high performance ion chromatography[J]. Chemical Geology, 2006, 227(1-2): 26−36. doi: 10.1016/j.chemgeo.2005.05.012
[20] 贺茂勇, 逯海, 金章东, 等. 人牙齿中锶的特效树脂分离及其同位素测定[J]. 分析化学, 2012, 40(7): 1109−1113.
He M Y, Lu H, Jin Z D, et al. Separation and isotopic measurement of Sr in tooth samples using selective specific resins[J]. Chinese Journal of Analytical Chemistry, 2012, 40(7): 1109−1113.
[21] Li C F, Li X H, Li Q L, et al. Rapid and precise determination of Sr and Nd isotopic ratios in geological samples from the same filament loading by thermal ionization mass spectrometry employing a single-step separation scheme[J]. Analytica Chimica Acta, 2012, 727: 54−60. doi: 10.1016/j.aca.2012.03.040
[22] 刘文刚, 刘卉, 李国占, 等. 离子交换树脂在地质样品Sr-Nd同位素测定中的应用[J]. 地质学报, 2017, 91(11): 2584−2592. doi: 10.3969/j.issn.0001-5717.2017.11.013
Liu W G, Liu H, Li G Z, et al. The apllication of ion exchange resins in Sr-Nd isotopic assay of geological samples[J]. Acta Geologica Sinica, 2017, 91(11): 2584−2592. doi: 10.3969/j.issn.0001-5717.2017.11.013
[23] Yang Y H, Wu F Y, Liu Z C, et al. Evaluation of Sr chemical purification technique for natural geological samples using common cation-exchange and Sr-specific extraction chromatographic resin prior to MC-ICP-MS or TIMS measurement[J]. Journal of Analytical Atomic Spectrometry, 2012, 27(3): 516−522. doi: 10.1039/c2ja10333h
[24] Horwitz E P, Dietz M L, Fisher D E. Separation and preconcentration of strontium from biological, environmental, and nuclear waste samples by extraction chromatography using a crown ether[J]. Analytical Chemistry, 1991, 63(5): 522−525. doi: 10.1021/ac00005a027
[25] Pin C, Gannoun A, Dupont A. Rapid, simultaneous separation of Sr, Pb, and Nd by extraction chromatography prior to isotope ratios determination by TIMS and MC-ICP-MS[J]. Journal of Analytical Atomic Spectrometry, 2014, 29(10): 1858−1870. doi: 10.1039/C4JA00169A
[26] 徐卓, 李力力, 朱留超, 等. Eichrom Sr树脂用于铀矿浓缩物中铅锶的分离富集研究[J]. 岩矿测试, 2019, 38(1): 55−61.
Xu Z, Li L L, Zhu L C, et al. Application of Eichrom Sr resin to the separation and enrichment of lead and strontium in uranium ore concentrates[J]. Rock and Mineral Analysis, 2019, 38(1): 55−61.
[27] Misawa K, Yamazaki F, Ihira N, et al. Separation of rare earth elements and strontium from chondritic meteorites by miniaturized extraction chromatography for elemental and isotopic analyses[J]. Geochemical Journal, 2000, 34(1): 11−21. doi: 10.2343/geochemj.34.11
[28] Smet I, Muynck D D, Vanhaecke F, et al. From volcanic rock powder to Sr and Pb isotope ratios: A fit-for-purpose procedure for multi-collector ICP-mass spectrometric analysis[J]. Journal of Analytical Atomic Spectrometry, 2010, 25(7): 1025−1032. doi: 10.1039/b926335g
[29] Li C F, Chu Z Y, Guo J H, et al. A rapid single column separation scheme for high-precision Sr-Nd-Pb isotopic analysis in geological samples using thermal ionization mass spectrometry[J]. Analytical Methods, 2015, 7(11): 4793−4802. doi: 10.1039/C4AY02896A
[30] Wieser M E, Buhl D, Bouman C, et al. High precision calcium isotope ratio measurements using a magnetic sector multiple collector inductively coupled plasma mass spectrometer[J]. Journal of Analytical Atomic Spectrometry, 2004, 19(7): 844−851. doi: 10.1039/b403339f
[31] Ludwig K R. Optimization of multicollector isotope-ratio measurement of strontium and neodymium[J]. Chemical Geology, 1997, 135(3-4): 325−334. doi: 10.1016/S0009-2541(96)00120-9
[32] Holmden C, Bélanger N. Ca isotope cycling in a forested ecosystem[J]. Geochimica et Cosmochimica Acta, 2010, 74(3): 995−1015. doi: 10.1016/j.gca.2009.10.020
[33] Wang Y, He Y, Wang Z N, et al. High-precision calcium isotope analysis on TIMS using a double spike technique: The instrumental drift and its correction[J]. Atomic Spectroscopy, 2023, 44(1): 45−54.
[34] Yang Y H, Zhang H F, Chu Z Y, et al. Combined chemical separation of Lu, Hf, Rb, Sr, Sm and Nd from a single rock digest and precise and accurate isotope determinations of Lu-Hf, Rb-Sr and Sm-Nd isotope systems using multi-collector ICP-MS and TIMS[J]. International Journal of Mass Spectrometry, 2010, 290(2): 120−126.
[35] 杨林, 石震, 于慧敏, 等. 多接收电感耦合等离子体质谱法测定岩石和土壤等国家标准物质的硅同位素组成[J]. 岩矿测试, 2023, 42(1): 136−145. doi: 10.3969/j.issn.0254-5357.2023.1.ykcs202301010
Yang L, Shi Z, Yu H M, et al. Determination of silicon isotopic compositions of rock and soil reference materials by MC-ICP-MS[J]. Rock and Mineral Analysis, 2023, 42(1): 136−145. doi: 10.3969/j.issn.0254-5357.2023.1.ykcs202301010
[36] Liu F, Li X, Zhang Z, et al. Calcium isotope ratios ( δ44/40Ca) of thirty-four geological Chinese reference materials measured by thermal ionisation mass spectrometry (TIMS)[J]. Geostandards and Geoanalytical Research, 2022, 46(2): 307−319. doi: 10.1111/ggr.12418
[37] Wu G, Zhu J M, Wang X, et al. High-sensitivity measurement of Cr isotopes by double spike MC-ICP-MS at the 10ng level[J]. Analytical Chemistry, 2020, 92(1): 1463−1469. doi: 10.1021/acs.analchem.9b04704
[38] Chen X Q, Zeng Z, Yu H M, et al. Precise measurements of δ88/86Sr for twenty geological reference materials by double-spike MC-ICP-MS[J]. International Journal of Mass Spectrometry, 2022, 479: 116883. doi: 10.1016/j.ijms.2022.116883
[39] Fourny A, Weis D, Scoates J S. Comprehensive Pb-Sr-Nd-Hf isotopic, trace element, and mineralogical characterization of mafic to ultramafic rock reference materials[J]. Geochemistry, Geophysics, Geosystems, 2016, 17(3): 739−773. doi: 10.1002/2015GC006181
[40] Wu S, Yang Y, Jochum K P, et al. Isotopic compositions (Li-B-Si-O-Mg-Sr-Nd-Hf-Pb) and Fe2+/ΣFe ratios of three synthetic andesite glass reference materials (ARM-1, ARM-2, ARM-3)[J]. Geostandards and Geoanalytical Research, 2021, 45(4): 719−745. doi: 10.1111/ggr.12399
[41] Zhang Z, Ma J, Zhang L, et al. Rubidium purification via a single chemical column and its isotope measurement on geological standard materials by MC-ICP-MS[J]. Journal of Analytical Atomic Spectrometry, 2018, 33(2): 322−328. doi: 10.1039/C7JA00406K
-



 下载:
下载: