Distribution pattern and controlling factors of redox sensitive elements in the surface sediments from four typical transects in the South China Sea
-
摘要:
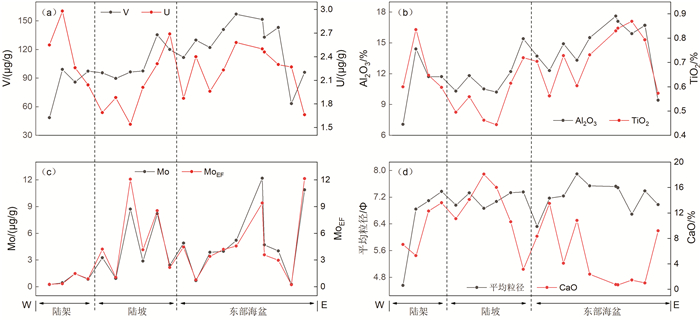
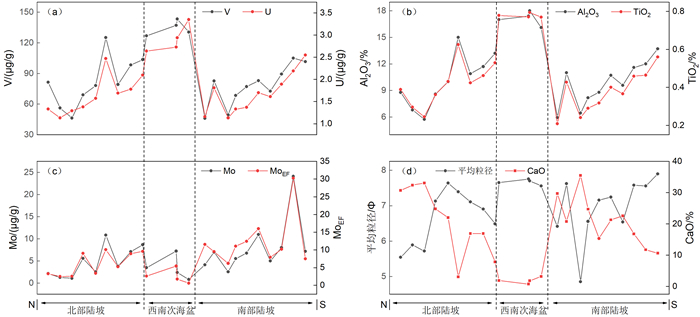
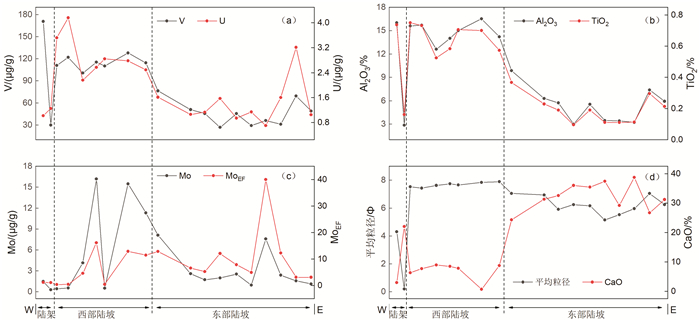
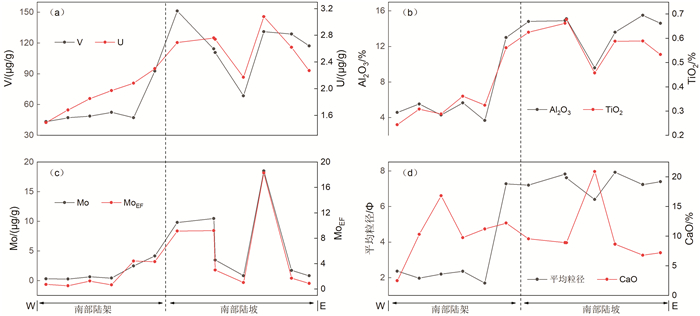
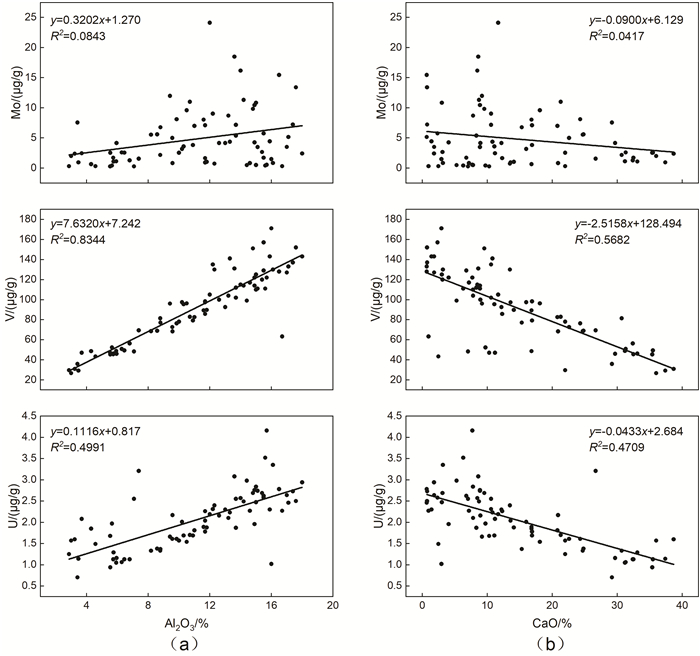
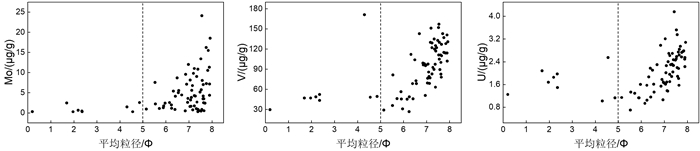
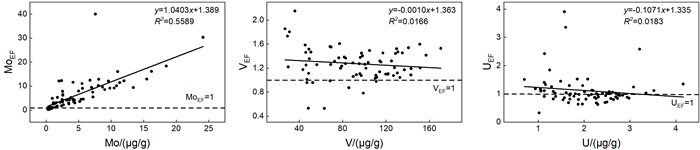
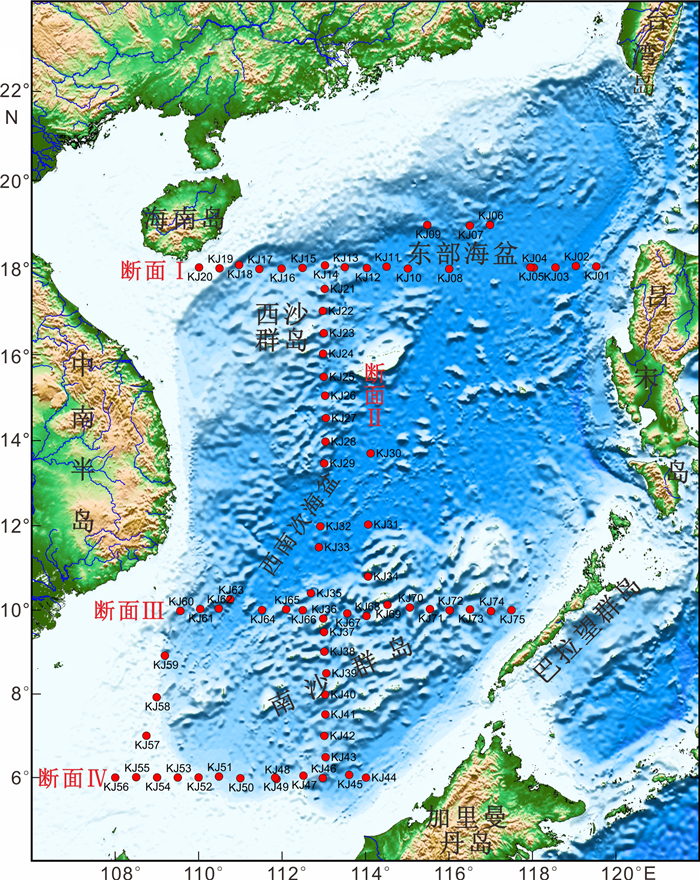
氧化还原敏感元素在环境研究中发挥着日益重要的作用,然而对于海底表层沉积物中氧化还原敏感元素的分布规律与特征的研究鲜有涉及。本文以采集自南海4条典型断面(18°N、10°N、6°N、113°E)的75个表层沉积物样品为研究对象,通过主量元素和微量元素(含氧化还原敏感元素Mo、V、U)分析,并结合沉积物粒度、元素富集系数等数据,探讨了表层沉积物中氧化还原敏感元素的分布特征及其控制因素。结果表明,研究区每个断面中的V、U含量变化趋势十分相似,Mo含量变化与V、U的总体变化趋势相近,但Mo在断面上的变化波动比V、U更强烈。4条断面中Mo平均含量表现出明显富集,除了V在断面Ⅰ中表现为轻度富集外,V和U平均含量都表现为亏损。影响沉积物中Mo、V、U含量分布的因素主要包括陆源碎屑含量、生物碳酸盐含量、细粒沉积物的吸附作用和氧化还原环境等。所有断面中V和U的含量分布主要受控于陆源碎屑组分,同时也受到生物碳酸盐含量和细粒沉积物的吸附作用的影响,氧化还原环境对其含量影响较小,受环境影响的自生组分含量较低。Mo的含量分布主要受控于海底氧化还原环境,陆源碎屑组分的贡献和细粒沉积物吸附作用的影响较小,受环境影响的自生组分含量较高。西南次海盆的Mo含量及其富集系数都较低,可能是由于西南次海盆的底流活动使其海底存在氧化环境所致。
Abstract:Redox sensitive elements play an increasingly important role in environmental analysis. However, few studies have been devoted so far to the distribution pattern of the redox sensitive elements in the seafloor surface sediments. In this paper, seventy-five surface sediment samples were collected and analyzed from the four representative transects in the South China Sea along 18°N, 10°N, 6°N, and 113°E respectively. The contents of main elements and trace elements (including Mo, V, and U) are measured in addition to grain sizes of sediments and enrichment factors of redox sensitive elements. The distribution pattern and controlling factors of redox sensitive elements are then discussed in the paper. Results show that the variations in V and U contents in each transect are in fact very similar. The content variation of Mo is similar to the overall trends of V and U, but more intense than V and U changes. Obviously, the average content of Mo is enriched while V and U depleted in the transects except slightly enriched V in the transect Ⅰ. The contents of V and U in all transects are mainly controlled by the contents of terrigenous debris and biological carbonate as well as the adsorption of fine-grained sediments, whereas the influence of redox environment is low. In contrast, the distribution of Mo mainly depends on the seabed redox environment, but not the contribution of terrigenous debris content and the adsorption of fine-grained sediments. The lower content and enrichment factor of Mo in the Southwestern Sub-basin of the South China Sea may probably attribute to oxidized environment caused by the underflow activity.
-
Key words:
- redox sensitive elements /
- controlling factors /
- surface sediments /
- typical transects /
- South China Sea
-

-
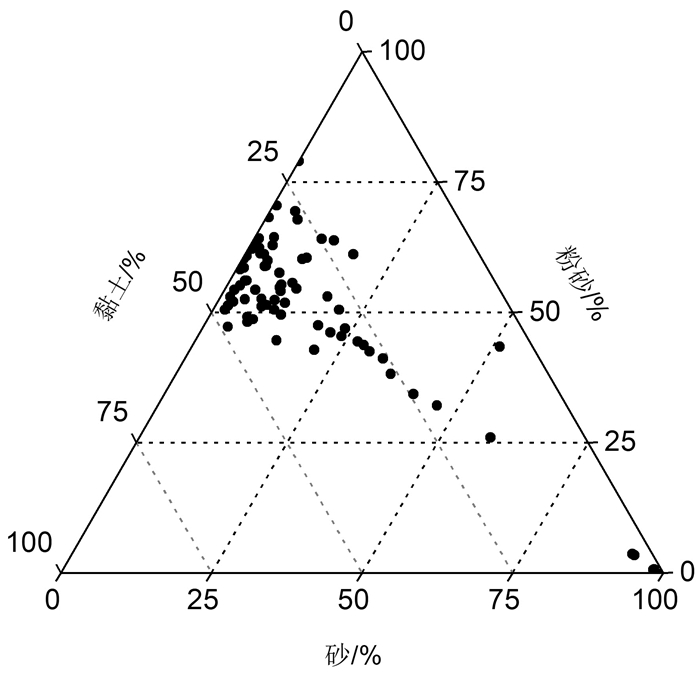
表 1 南海典型断面表层沉积物粒级组成、平均粒径、微量元素(含氧化还原敏感元素)、主量元素分析结果和氧化还原敏感元素富集系数
Table 1. Grain size composition, mean grain size, contents of trace elements (including redox sensitive elements) and main elements, and the enrichment factors of redox sensitive elements of the surface sediments from four typical transects in the South China Sea
样品号 砾 砂 粉砂 黏土 平均
粒径/ΦMo V U Sr Al2O3 TiO2 CaO Fe2O3 MnO MoEF VEF UEF /% /(μg/g) /% KJ01 0.00 10.55 60.47 28.99 6.98 12.0 96.0 1.66 589 9.41 0.57 9.19 3.56 0.06 12.15 1.10 0.68 KJ02 0.00 0.38 68.22 31.40 7.39 0.29 63.3 2.27 335 16.7 0.79 0.98 6.68 0.60 0.21 0.53 0.68 KJ03 0.00 0.00 79.02 20.98 6.69 4.42 143 2.30 134 15.9 0.87 1.43 6.38 0.65 2.96 1.09 0.63 KJ04 0.00 0.81 64.17 35.01 7.48 5.16 133 2.46 137 17.1 0.84 0.69 6.99 1.67 3.57 1.04 0.69 KJ05 0.00 1.76 62.39 35.85 7.52 13.4 152 2.50 129 17.6 0.83 0.74 7.14 0.89 9.39 1.20 0.71 KJ06 0.00 5.12 54.33 40.55 7.53 5.73 157 2.58 136 15.5 0.73 2.36 6.50 1.01 4.56 1.42 0.84 KJ07 0.00 1.61 52.98 45.42 7.89 4.35 141 2.23 176 13.3 0.60 10.8 5.23 1.49 4.19 1.54 0.88 KJ08 0.00 3.22 64.38 32.40 7.23 4.26 122 1.96 413 14.9 0.73 4.08 5.81 0.23 3.41 1.11 0.64 KJ09 0.00 8.93 54.81 36.26 7.17 0.74 130 2.40 221 12.3 0.56 13.5 4.73 1.10 0.77 1.52 1.01 KJ10 0.00 13.45 63.79 22.77 6.32 5.38 112 1.87 516 13.7 0.70 8.34 5.23 0.57 4.45 1.05 0.63 KJ11 0.00 4.58 58.92 36.50 7.35 2.67 120 2.69 343 15.4 0.72 3.12 5.93 1.60 2.16 1.10 0.89 KJ12 0.00 4.46 58.84 36.70 7.33 9.01 135 2.31 205 12.3 0.61 10.6 4.74 0.74 8.54 1.45 0.89 KJ13 0.00 8.99 55.30 35.71 7.07 3.17 97.5 2.01 401 10.2 0.44 16.0 3.66 1.86 4.14 1.45 1.07 KJ14 0.00 9.95 60.23 29.83 6.86 9.58 96.3 1.54 611 10.5 0.46 18.1 3.76 1.05 12.07 1.38 0.79 KJ15 0.00 3.18 61.18 35.64 7.33 1.02 89.7 1.88 522 11.8 0.56 14.1 4.29 0.34 1.06 1.06 0.80 KJ16 0.00 5.38 67.82 26.80 6.96 3.59 95.4 1.69 449 10.3 0.49 11.1 3.72 0.31 4.22 1.27 0.81 KJ17 0.00 4.31 59.95 35.74 7.37 0.91 97.3 2.04 528 11.7 0.60 13.6 4.15 0.23 0.89 1.08 0.81 KJ18 0.00 0.57 70.50 28.94 7.09 1.63 85.7 2.26 462 11.7 0.65 12.3 4.31 0.33 1.47 0.87 0.83 KJ19 0.00 4.17 69.39 26.44 6.84 0.48 99.0 2.98 233 14.4 0.83 5.28 5.42 0.09 0.34 0.78 0.85 KJ20 0.00 58.32 26.01 15.67 4.55 0.28 48.3 2.55 314 7.07 0.60 7.04 3.60 0.07 0.27 0.53 1.01 KJ21 0.00 30.00 42.47 27.54 5.54 2.18 81.4 1.33 890 8.78 0.39 30.7 3.16 0.39 3.27 1.39 0.82 KJ22 0.00 27.10 44.39 28.51 5.89 1.28 56.3 1.13 920 6.79 0.30 32.4 2.57 0.23 2.53 1.26 0.91 KJ23 0.00 32.87 41.14 25.99 5.72 1.07 46.2 1.29 1450 5.74 0.24 33.1 2.11 0.20 2.54 1.25 1.25 KJ24 0.00 11.30 51.80 36.90 7.13 5.59 68.9 1.38 802 8.58 0.36 24.8 3.14 0.89 9.02 1.26 0.91 KJ25 0.00 4.26 52.53 43.21 7.64 2.52 78.1 1.57 681 9.98 0.43 22.0 3.78 0.72 3.40 1.20 0.86 KJ26 0.00 7.03 52.57 40.40 7.39 10.8 125 2.47 192 15.0 0.63 3.03 5.53 2.60 10.09 1.32 0.94 KJ27 0.00 11.76 49.51 38.73 7.11 3.81 79.3 1.69 579 10.9 0.42 16.9 3.72 0.57 5.24 1.23 0.95 KJ28 0.00 11.86 54.53 33.61 6.90 7.10 98.4 1.78 601 11.7 0.46 16.9 3.95 0.80 8.93 1.40 0.92 KJ29 0.00 11.20 64.15 24.66 6.49 8.68 104 2.10 345 13.2 0.53 7.84 4.71 1.47 9.55 1.29 0.94 KJ30 0.00 0.37 60.89 38.74 7.65 3.50 127 2.64 139 17.0 0.78 1.84 6.55 0.53 2.61 1.07 0.80 KJ31 0.00 0.61 58.24 41.15 7.75 7.22 137 2.73 120 17.4 0.77 0.75 6.69 0.93 5.45 1.17 0.84 KJ32 0.00 0.20 60.29 39.51 7.70 2.39 143 2.94 144 18.0 0.79 1.80 6.86 0.41 1.75 1.19 0.88 KJ33 0.00 0.11 63.06 36.83 7.56 0.83 130 3.35 183 16.1 0.77 3.19 6.10 0.11 0.63 1.12 1.03 KJ34 0.00 17.76 53.02 29.22 6.41 4.14 46.0 1.16 1063 5.94 0.21 29.7 2.02 1.06 11.58 1.46 1.33 KJ35 0.00 6.36 49.14 44.51 7.62 6.99 82.6 1.81 660 11.0 0.43 20.8 3.96 1.26 9.51 1.27 1.00 KJ36 0.00 46.37 32.15 21.49 4.85 2.56 49.4 1.13 1006 6.45 0.24 35.5 2.35 0.41 6.19 1.35 1.11 KJ37 0.00 18.96 47.48 33.56 6.55 5.52 68.5 1.33 915 8.18 0.29 24.7 2.68 0.71 11.06 1.56 1.09 KJ38 0.00 13.43 44.67 41.90 7.15 6.77 77.2 1.37 571 8.79 0.32 15.3 2.93 0.77 12.43 1.61 1.02 KJ39 0.00 8.44 51.42 40.14 7.24 11.0 82.8 1.70 710 10.7 0.40 21.3 3.70 1.54 16.00 1.37 1.01 KJ40 0.00 20.66 42.81 36.53 6.54 4.99 72.7 1.61 724 9.57 0.37 22.6 3.36 0.80 7.94 1.31 1.04 KJ41 0.00 6.89 48.14 44.98 7.58 8.03 89.3 1.89 587 11.6 0.46 16.8 4.14 1.08 10.19 1.29 0.98 KJ42 0.00 7.54 48.71 43.75 7.56 24.1 105 2.19 497 12.0 0.46 11.7 3.94 3.00 30.26 1.49 1.12 KJ43 0.00 4.12 47.22 48.66 7.90 7.17 102 2.55 434 13.7 0.56 10.6 4.83 1.96 7.46 1.20 1.08 KJ44 0.00 7.71 51.14 41.15 7.40 0.82 117 2.27 342 14.6 0.53 7.18 4.93 0.21 0.89 1.45 1.01 KJ45 0.00 7.51 57.61 34.88 7.24 1.71 129 2.62 331 15.5 0.59 6.79 5.13 0.36 1.69 1.44 1.06 KJ46 0.00 1.95 50.48 47.57 7.92 18.5 131 3.08 425 13.6 0.59 8.63 4.71 3.77 18.29 1.47 1.24 KJ47 0.00 21.68 46.16 32.17 6.39 0.81 68.4 2.17 1002 9.58 0.46 20.9 3.65 0.12 1.03 0.99 1.13 KJ48 0.00 2.57 56.04 41.39 7.61 3.47 111 2.74 384 15.1 0.68 8.88 5.48 1.25 2.98 1.08 0.96 KJ49 0.00 1.63 54.37 44.01 7.82 10.5 114 2.76 385 14.9 0.66 8.89 5.31 1.57 9.19 1.14 0.99 KJ50 0.00 3.68 62.91 33.41 7.20 9.81 151 2.69 425 14.8 0.62 9.54 4.91 2.49 9.14 1.60 1.02 KJ51 0.00 10.17 50.50 39.34 7.27 4.15 92.6 2.30 437 13.0 0.56 12.2 4.86 0.82 4.30 1.09 0.97 KJ52 0.00 98.11 0.69 1.20 1.69 2.46 47.1 2.08 429 3.67 0.32 11.2 3.85 0.33 4.41 0.96 1.52 KJ53 0.00 93.03 3.63 3.34 2.35 0.41 52.4 1.97 375 5.64 0.36 9.74 3.85 0.08 0.66 0.96 1.29 KJ54 0.00 100.00 0.00 0.00 2.19 0.62 48.6 1.85 610 4.29 0.29 16.8 4.70 0.47 1.26 1.11 1.52 KJ55 0.00 98.04 0.60 1.36 1.97 0.27 47.1 1.68 509 5.52 0.31 10.3 3.21 0.06 0.51 1.01 1.29 KJ56 0.00 93.51 3.38 3.11 2.36 0.31 43.4 1.49 123 4.57 0.24 2.47 3.15 0.05 0.73 1.17 1.45 KJ57 0.00 51.14 43.40 5.46 4.30 1.47 171 1.02 200 16.0 0.74 2.96 7.82 0.31 1.16 1.53 0.33 KJ58 36.65 55.15 7.15 1.06 0.20 0.29 29.7 1.25 963 2.85 0.16 22.0 2.53 0.17 1.04 1.22 1.84 KJ59 0.00 0.64 62.17 37.20 7.53 0.41 111 3.52 285 15.6 0.75 6.31 5.95 0.08 0.32 0.98 1.11 KJ60 0.00 2.49 61.28 36.23 7.43 0.54 122 4.16 342 15.7 0.73 7.72 5.34 0.06 0.43 1.10 1.35 KJ61 0.00 2.77 56.08 41.15 7.62 4.15 100 2.16 399 12.6 0.52 8.96 4.24 1.25 4.60 1.26 0.98 KJ62 0.00 2.19 55.12 42.69 7.75 16.2 115 2.57 379 14.0 0.58 8.48 4.66 1.79 16.12 1.30 1.04 KJ63 0.00 1.10 58.55 40.35 7.66 0.49 110 2.84 330 15.0 0.71 7.82 5.74 0.10 0.41 1.03 0.95 KJ64 0.00 2.55 52.09 45.36 7.84 15.5 128 2.78 129 16.5 0.70 0.69 6.21 2.19 12.86 1.21 0.94 KJ65 0.00 2.11 51.28 46.61 7.89 11.3 114 2.49 379 14.2 0.57 8.74 5.23 2.35 11.48 1.32 1.03 KJ66 0.00 9.42 54.10 36.49 7.05 8.10 76.4 1.61 739 9.85 0.37 24.3 3.26 0.58 12.84 1.37 1.04 KJ67 0.00 10.58 55.66 33.76 6.94 2.58 50.8 1.06 907 6.28 0.23 31.3 2.28 0.36 6.56 1.47 1.10 KJ68 0.00 28.46 43.71 27.84 5.91 1.72 45.5 1.13 1181 5.72 0.19 32.5 1.85 0.52 5.26 1.58 1.41 KJ69 0.00 20.88 50.51 28.61 6.25 1.98 26.8 1.57 4690 2.99 0.10 36.0 0.94 0.33 12.06 1.85 3.91 KJ70 0.00 23.74 46.94 29.33 6.16 2.54 45.4 0.94 1115 5.53 0.19 35.5 1.95 0.48 7.82 1.59 1.18 KJ71 0.00 41.37 34.32 24.31 5.15 0.94 29.2 1.14 3169 3.46 0.11 37.4 1.19 0.18 4.90 1.73 2.42 KJ72 0.00 35.62 38.18 26.20 5.53 7.57 35.9 0.70 1048 3.40 0.11 29.2 1.09 1.11 40.01 2.15 1.51 KJ73 0.00 17.99 61.10 20.91 5.96 2.38 31.0 1.60 2391 3.22 0.11 38.7 1.20 0.45 12.22 1.80 3.36 KJ74 0.00 9.34 52.33 38.33 7.04 1.56 69.4 3.21 1294 7.38 0.29 26.7 2.78 0.40 3.07 1.55 2.58 KJ75 0.00 23.85 45.41 30.74 6.25 1.13 48.8 1.05 1083 5.92 0.21 31.2 2.16 0.57 3.08 1.51 1.16 表 2 南海典型断面表层沉积物粒度、主量元素和氧化还原敏感元素含量及其特征参数
Table 2. Character parameters of grain sizes, contents of main elements and redox sensitive elements of the surface sediments from four typical transects in the South China Sea
断面 特征参数 粒级组分含量/% 氧化还原敏感元素/(μg/g) 主量元素/% 砾 砂 粉砂 黏土 Mo V U Al2O3 TiO2 CaO Fe2O3 MnO 断面Ⅰ 最小值 0.00 0.00 26.01 15.67 0.28 48.3 1.54 7.07 0.44 0.69 3.56 0.06 最大值 0.00 58.32 79.02 45.42 13.4 157 2.98 17.6 0.87 18.1 7.14 1.86 平均值 0.00 7.49 60.64 31.88 4.40 111 2.21 13.1 0.66 8.17 5.09 0.75 标准偏差 0.00 12.23 10.07 6.83 3.80 28.1 0.36 2.77 0.13 5.42 1.18 0.56 断面Ⅱ 最小值 0.00 0.11 32.15 21.49 0.83 46.0 1.13 5.74 0.21 0.75 2.02 0.11 最大值 0.00 46.37 64.15 48.66 24.1 143 3.35 18.0 0.79 35.5 6.86 3.00 平均值 0.00 13.01 50.45 36.54 6.01 89.1 1.88 11.2 0.46 17.6 4.03 0.98 标准偏差 0.00 11.59 7.50 7.13 4.82 28.2 0.62 3.61 0.18 10.8 1.43 0.72 断面Ⅲ 最小值 0.00 0.64 7.15 1.06 0.29 26.8 0.70 2.85 0.10 0.69 0.94 0.06 最大值 36.65 55.15 62.17 46.61 16.2 171 4.16 16.5 0.75 38.7 7.82 2.35 平均值 1.93 17.97 48.91 31.19 4.25 76.8 1.94 9.27 0.39 20.9 3.50 0.70 标准偏差 8.18 17.05 12.37 11.86 4.91 41.9 0.98 5.16 0.25 13.0 2.03 0.69 断面Ⅳ 最小值 0.00 1.63 0.00 0.00 0.27 43.4 1.49 3.67 0.24 2.47 3.15 0.05 最大值 0.00 100.00 62.91 47.57 18.5 151 3.08 15.5 0.68 20.9 5.48 3.77 平均值 0.00 41.51 33.65 24.84 4.14 88.7 2.29 10.4 0.48 10.3 4.44 0.89 标准偏差 0.00 43.82 25.60 18.66 5.30 37.3 0.45 4.69 0.15 4.37 0.76 1.09 全海区 最小值 0.00 0.00 0.00 0.00 0.27 26.8 0.70 2.85 0.10 0.69 0.94 0.05 最大值 36.65 100.00 79.02 48.66 24.1 171 4.16 18.0 0.87 38.7 7.82 3.77 平均值 0.49 17.73 49.87 31.91 4.81 91.7 2.05 11.1 0.50 14.6 4.25 0.83 标准偏差 4.20 24.91 16.54 11.80 4.76 36.0 0.68 4.31 0.21 10.8 1.58 0.76 表 3 南海典型断面表层沉积物中主量和微量元素相关系数
Table 3. Correlation coefficients of main and trace elements of the surface sediments from four typical transects in the South China Sea
Mo V U Sr Al2O3 TiO2 CaO Fe2O3 MnO Mo 1.00 V 0.36 1.00 U 0.13 0.63 1.00 Sr -0.18 -0.62 -0.43 1.00 Al2O3 0.29 0.91 0.71 -0.63 1.00 TiO2 0.17 0.86 0.74 -0.67 0.94 1.00 CaO -0.20 -0.75 -0.69 0.74 -0.79 -0.87 1.00 Fe2O3 0.16 0.86 0.68 -0.70 0.91 0.94 -0.89 1.00 MnO 0.78 0.39 0.23 -0.21 0.33 0.18 -0.21 0.21 1.00 -
[1] Morford J L, Russell A D, Emerson S. Trace metal evidence for changes in the redox environment associated with the transition from terrigenous clay to diatomaceous sediment, Saanich Inlet, BC[J]. Marine Geology, 2001, 174(1-4): 355-369. doi: 10.1016/S0025-3227(00)00160-2
[2] 常华进, 储雪蕾, 冯连君, 等.氧化还原敏感微量元素对古海洋沉积环境的指示意义[J].地质论评, 2009, 55(1): 91-99. doi: 10.3321/j.issn:0371-5736.2009.01.011
CHANG Huajin, CHU Xuelei, FENG Lianjun, et al. Redox sensitive trace elements as paleoenvironments proxies[J]. Geological Review, 2009, 55(1): 91-99. doi: 10.3321/j.issn:0371-5736.2009.01.011
[3] Rimmer S M. Geochemical paleoredox indicators in Devonian-Mississippian black shales, central Appalachian basin (USA)[J]. Chemical Geology, 2004, 206(3-4): 373-391. doi: 10.1016/j.chemgeo.2003.12.029
[4] 许淑梅, 翟世奎, 张爱滨, 等.长江口及其邻近海域表层沉积物中氧化还原敏感性微量元素的环境指示意义[J].沉积学报, 2007, 25(5): 759-766. doi: 10.3969/j.issn.1000-0550.2007.05.015
XU Shumei, ZHAI Shikui, ZHANG Aibin, et al. Distribution and environment significance of redox sensitive trace elements of the Changjiang Estuary Hypoxia Zone and its contiguous sea area[J]. Acta Sedimentologica Sinica, 2007, 25(5): 759-766. doi: 10.3969/j.issn.1000-0550.2007.05.015
[5] 孟楚洁, 胡文瑄, 贾东, 等.宁镇地区上奥陶统五峰组——下志留统高家边组底部黑色岩系地球化学特征与沉积环境分析[J].地学前缘, 2017, 24(6): 300-311. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dxqy201706023
MENG Chujie, HU Wenxuan, JIA Dong, et al. Analyses of geochemistry features and sedimentary environment in the Upper Ordovician Wufeng-Lower Silurian Gaojiabian Formations in Nanjing-Zhenjiang area[J]. Earth Science Frontiers, 2017, 24(6): 300-311. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dxqy201706023
[6] 王双, 杨瑞东.贵阳花溪燕楼剖面下三叠统大冶组地球化学特征与沉积环境分析[J].古地理学报, 2018, 20(2): 285-298. http://d.old.wanfangdata.com.cn/Periodical/gdlxb201802009
WANG Shuang, YANG Ruidong. Analysis of geochemistry features and sedimentary environment of the Lower Triassic Daye Formation in Yanlou section of Huaxi, Guiyang[J]. Journal of Palaeogeography, 2018, 20(2): 285-298. http://d.old.wanfangdata.com.cn/Periodical/gdlxb201802009
[7] Algeo T J, Maynard J B. Trace-metal covariation as a guide to water-mass conditions in ancient anoxic marine environments[J]. Geosphere, 2008, 4(5): 872-887. doi: 10.1130/GES00174.1
[8] Algeo T J, Tribovillard N. Environmental analysis of paleoceanographic systems based on molybdenum-uranium covariation[J]. Chemical Geology, 2009, 268(3-4): 211-225. doi: 10.1016/j.chemgeo.2009.09.001
[9] Tribovillard N, Algeo T J, Baudin F, et al. Analysis of marine environmental conditions based on molybdenum-uranium covariation-Applications to Mesozoic paleoceanography[J]. Chemical Geology, 2012, 324: 46-58.
[10] Algeo T J, Morford J, Cruse A. Reprint of: New applications of trace metals as proxies in marine paleoenvironments[J]. Chemical Geology, 2012, 324: 1-5.
[11] 汤冬杰, 史晓颖, 赵相宽, 等. Mo-U共变作为古沉积环境氧化还原条件分析的重要指标——进展、问题与展望[J].现代地质, 2015, 29(1): 1-13. doi: 10.3969/j.issn.1000-8527.2015.01.001
TANG Dongjie, SHI Xiaoying, ZHAO Xiangkuan, et al. Mo-U Covariation as an important proxy for sedimentary environment redox conditions-progress, problems and prospects[J]. Geoscience, 2015, 29(1): 1-13. doi: 10.3969/j.issn.1000-8527.2015.01.001
[12] Dahl T W, Anbar A D, Gordon G W, et al. The behavior of molybdenum and its isotopes across the chemocline and in the sediments of sulfidic Lake Cadagno, Switzerland[J]. Geochimica et Cosmochimica Acta, 2010, 74(1): 144-163. doi: 10.1016/j.gca.2009.09.018
[13] 温汉捷, 张羽旭, 樊海峰, 等.华南下寒武统地层的Mo同位素组成特征及其古海洋环境意义[J].科学通报, 2010, 55(2): 176-181. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kxtb201002009
WEN Hanjie, ZHANG Yuxu, FAN Haifeng, et al. Mo isotopes in the Lower Cambrian formation of southern China and its implications on paleo-ocean environment[J]. Chinese Science Bulletin, 2010, 55(2): 176-181. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kxtb201002009
[14] 周炼, 苏洁, 黄俊华, 等.判识缺氧事件的地球化学新标志——钼同位素[J].中国科学:地球科学, 2011, 41(3): 309-319. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgkx-cd201103004
ZHOU Lian, SU Jie, HUANG Junhua, et al. A new paleoenvironmental index for anoxic events-Mo isotopes in black shales from Upper Yangtze marine sediments[J]. Science China Earth Science, 2011, 41(3): 309-319. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgkx-cd201103004
[15] Andersen M B, Romaniello S, Vance D, et al. A modern framework for the interpretation of U-238/U-235 in studies of ancient ocean redox[J]. Earth and Planetary Science Letters, 2014, 400: 184-194. doi: 10.1016/j.epsl.2014.05.051
[16] Dickson A J, Cohen A S, Coe A L. Continental margin molybdenum isotope signatures from the early Eocene[J]. Earth and Planetary Science Letters, 2014, 404: 389-395. doi: 10.1016/j.epsl.2014.08.004
[17] Wen H J, Fan H F, Zhang Y X, et al. Reconstruction of early Cambrian ocean chemistry from Mo isotopes[J]. Geochimica et Cosmochimica Acta, 2015, 164: 1-16. doi: 10.1016/j.gca.2015.05.008
[18] Kurzweil F, Wille M, Schoenberg R, et al. Continuously increasing delta Mo-98 values in Neoarchean black shales and iron formations from the Hamersley Basin[J]. Geochimica et Cosmochimica Acta, 2015, 164: 523-542. doi: 10.1016/j.gca.2015.05.009
[19] Goto K T, Anbar A D, Gordon G W, et al. Uranium isotope systematics of ferromanganese crusts in the Pacific Ocean: Implications for the marine U-238/U-235 isotope system[J]. Geochimica et Cosmochimica Acta, 2014, 146: 43-58. doi: 10.1016/j.gca.2014.10.003
[20] Algeo T J, Maynard J B. Trace-element behavior and redox facies in core shales of Upper Pennsylvanian Kansas-type cyclothems[J]. Chemical Geology, 2004, 206(3-4): 289-318. doi: 10.1016/j.chemgeo.2003.12.009
[21] Asael D, Rouxel O, Poulton S W, et al. Molybdenum record from black shales indicates oscillating atmospheric oxygen levels in the Early Paleoproterozoic[J]. American Journal of Science, 2018, 318(3): 275-299. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=44fc2fdab875978dffa324b6e29f5cf5
[22] Palomares R M, Hernandez R L, Frias J M. Mechanisms of trace metal enrichment in submarine, methane-derived carbonate chimneys from the Gulf of Cadiz[J]. Journal of Geochemical Exploration, 2012, 112: 297-305. doi: 10.1016/j.gexplo.2011.09.011
[23] Sato H, Hayashi K, Ogawa Y, et al. Geochemistry of deep sea sediments at cold seep sites in the Nankai Trough: Insights into the effect of anaerobic oxidation of methane[J]. Marine Geology, 2012, 323: 47-55. https://www.sciencedirect.com/science/article/abs/pii/S0025322712001648
[24] Hu Y, Feng D, Peckmann J, et al. New insights into cerium anomalies and mechanisms of trace metal enrichment in authigenic carbonate from hydrocarbon seeps[J]. Chemical Geology, 2014, 381: 55-66. doi: 10.1016/j.chemgeo.2014.05.014
[25] Adelson J M, Helz G R, Miller C V. Reconstructing the rise of recent coastal anoxia; molybdenum in Chesapeake Bay sediments[J]. Geochimica et Cosmochimica Acta, 2001, 65(2): 237-252. doi: 10.1016/S0016-7037(00)00539-1
[26] Chaillou G, Anschutz P, Lavaux G, et al. The distribution of Mo, U, and Cd in relation to major redox species in muddy sediments of the Bay of Biscay[J]. Marine Chemistry, 2002, 80(1): 41-59. http://d.old.wanfangdata.com.cn/NSTLQK/10.1016-S0304-4203(02)00097-X/
[27] Ge L, Jiang S Y, Swennen R, et al. Chemical environment of cold seep carbonate formation on the northern continental slope of South China Sea: Evidence from trace and rare earth element geochemistry[J]. Marine Geology, 2010, 277(1-4): 21-30. doi: 10.1016/j.margeo.2010.08.008
[28] Wang S H, Yan W, Chen Z, et al. Rare earth elements in cold seep carbonates from the southwestern Dongsha area, northern South China Sea[J]. Marine and Petroleum Geology, 2014, 57: 482-493. doi: 10.1016/j.marpetgeo.2014.06.017
[29] Wang S H, Zhang N, Chen H, et al. The surface sediment types and their rare earth element characteristics from the continental shelf of the northern south China sea[J]. Continental Shelf Research, 2014, 88: 185-202. doi: 10.1016/j.csr.2014.08.005
[30] Wang S H, Wu S Z, Yan W, et al. Rare metal elements in surface sediment from five bays on the northeastern coast of the South China Sea[J]. Environmental Earth Sciences, 2015, 74(6): 4961-4971. doi: 10.1007/s12665-015-4504-6
[31] Liu F W, Miao L, Cai G Q, et al. The rare earth element geochemistry of surface sediments in four transects in the South China Sea and its geological significance[J]. Environmental Earth Sciences, 2015, 74(3): 2511-2522. doi: 10.1007/s12665-015-4265-2
[32] McManus J. Grain size determination and interpretation[C]//In: Tucker M, ed. Techniques in sedimentology. Blackwell, Oxford, 1988: 63-85.
[33] Murray R W, Leinen M. Scavenged excess aluminum and its relationship to bulk titanium in biogenic sediment from the central equatorial Pacific Ocean[J]. Geochimica et Cosmochimica Acta, 1996, 60(20): 3869-3878. doi: 10.1016/0016-7037(96)00236-0
[34] Li G, Rashid H, Zhong L F, et al. Changes in deep water oxygenation of the South China Sea since the Last Glacial Period[J]. Geophysical Research Letters, 2018, 45. https://doi.org/10.1029/2018GL078568.
[35] Rudnick R L, Gao S. Composition of the Continental Crust[M]. Oxford: Elsevier, 2014: 1-51.
[36] 赵一阳.中国海大陆架沉积物地球化学的若干模式[J].地质科学, 1983, 18(4): 307-314. http://www.cnki.com.cn/Article/CJFDTOTAL-DZKX198304000.htm
ZHAO Yiyang. Some geochemical patterns of shelf sediments of the China Sea[J]. Scientia Geologica Sinica, 1983, 18(4): 307-314. http://www.cnki.com.cn/Article/CJFDTOTAL-DZKX198304000.htm
[37] 许淑梅, 翟世奎, 张爱滨, 等.长江口外缺氧区沉积物中元素分布的氧化还原环境效应[J].海洋地质与第四纪地质, 2007, 27(3): 1-8. http://hydz.chinajournal.net.cn/WKD/WebPublication/paperDigest.aspx?paperID=30eba64c-f181-4278-8516-cf40ad4a4e87
XU Shumei, ZHAI Shikui, ZHANG Aibin, et al. Redox environment effect on the redox sensitive elements in surface sediments from the Yangtze Estuary Hypoxia Zone[J]. Marine Geology & Quaternary Geology, 2007, 27(3): 1-8. http://hydz.chinajournal.net.cn/WKD/WebPublication/paperDigest.aspx?paperID=30eba64c-f181-4278-8516-cf40ad4a4e87
[38] Boning P, Brumsack H J, Bottcher M E, et al. Geochemistry of Peruvian near-surface sediments[J]. Geochimica et Cosmochimica Acta, 2004, 68(21): 4429-4451. doi: 10.1016/j.gca.2004.04.027
[39] 李泽文, 栾振东, 阎军, 等.南海北部外陆架表层沉积物粒度参数特征及物源分析[J].海洋科学, 2011, 35(12): 92-100. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hykx201112015
LI Zewen, LUAN Zhendong, YAN Jun, et al. Characterization of grain size parameters and the provenance analysis of the surface sediment in the outer shelf of the northern South China Sea[J]. Marine Sciences, 2011, 35(12): 92-100. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hykx201112015
[40] 张晋. 南海南部表层沉积物粒度和粘土矿物组成与分布特征及其物源指示[D]. 中国石油大学(华东)硕士学位论文, 2014.
http://cdmd.cnki.com.cn/Article/CDMD-10425-1016711605.htm ZHANG Jin. Composition and distribution characteristics of grain-size and clay minerals in surface sediments of the southern South China Sea and their indication to provenance[D]. Master's Thesis of China University of Petroleum(East China), 2014.
[41] 李学杰, 汪品先, 廖志良, 等.南海西部表层沉积物碎屑矿物分布特征及其物源[J].中国地质, 2008, (1): 123-130. doi: 10.3969/j.issn.1000-3657.2008.01.013
LI Xuejie, WANG Pinxian, LIAO Zhiliang, et al. Distribution of clastic minerals of surface sediments in the western China Sea and their provenance[J]. Geology in China, 2008, (1): 123-130. doi: 10.3969/j.issn.1000-3657.2008.01.013
[42] Zhang C S, Wang L J, Li G S, et al. Grain size effect on multi-element concentrations in sediments from the intertidal flats of Bohai Bay, China[J]. Applied Geochemistry, 2002, 17(1): 59-68. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=12899223c2a9304dd695642efb7bad9c
[43] 张晓东, 翟世奎, 许淑梅, 等.长江口外缺氧区沉积物中氧化还原敏感性元素的"粒控效应"[J].中国海洋大学学报:自然科学版, 2005, 35(5): 868-874. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=qdhydxxb200505034
ZHANG Xiaodong, ZHAI Shikui, XU Shumei, et al. The "Grain Size Effect" of redox sensitive elements in the sediments in the hypoxia zone of the Changjiang Estuary[J]. Periodical of Ocean University of China (Natural Science Edition), 2005, 35(5): 868-874. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=qdhydxxb200505034
[44] Tribovillard N, Algeo T J, Lyons T, et al. Trace metals as paleoredox and paleoproductivity proxies: An update[J]. Chemical Geology, 2006, 232(1-2): 12-32. doi: 10.1016/j.chemgeo.2006.02.012
[45] Erickson B E, Helz G R. Molybdenum (Ⅵ) speciation in sulfidic waters: Stability and lability of thiomolybdates[J]. Geochimica et Cosmochimica Acta, 2000, 64(7): 1149-1158. doi: 10.1016/S0016-7037(99)00423-8
[46] Wehrli B, Stumm W. Vanadyl in natural waters: Adsorption and hydrolysis promote oxygenation[J]. Geochimica et Cosmochimica Acta, 1989, 53(1): 69-77. doi: 10.1016/0016-7037(89)90273-1
[47] Breit G N, Wanty R B. Vanadium accumulation in carbonaceous rocks: A review of geochemical controls during deposition and diagenesis[J]. Chemical Geology, 1991, 91(2): 83-97. https://www.sciencedirect.com/science/article/abs/pii/0009254191900834
-



 下载:
下载: