Magma genesis and evolution of source composition during the weakening of Caroline mantle plume activity
-
摘要:
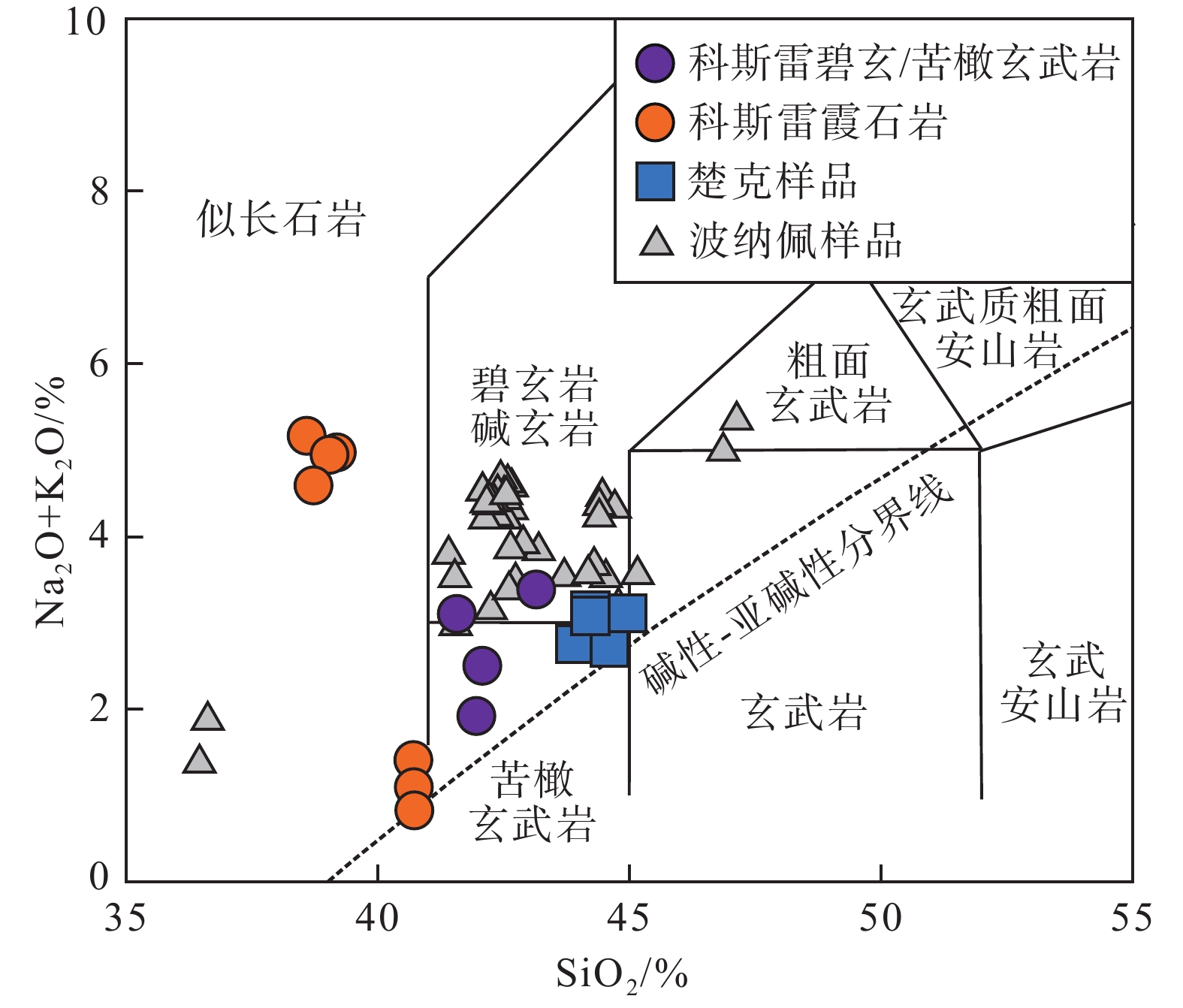
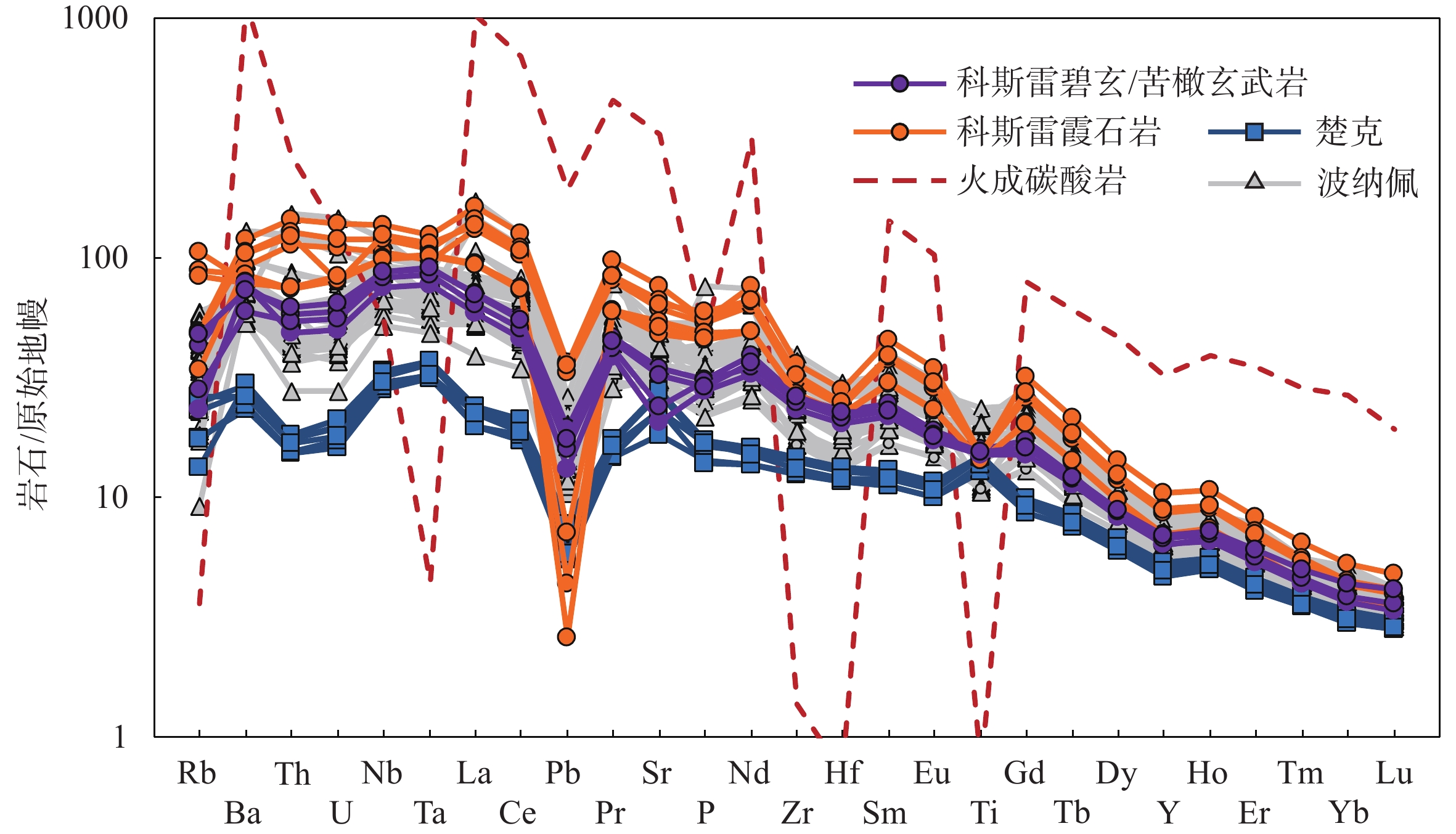
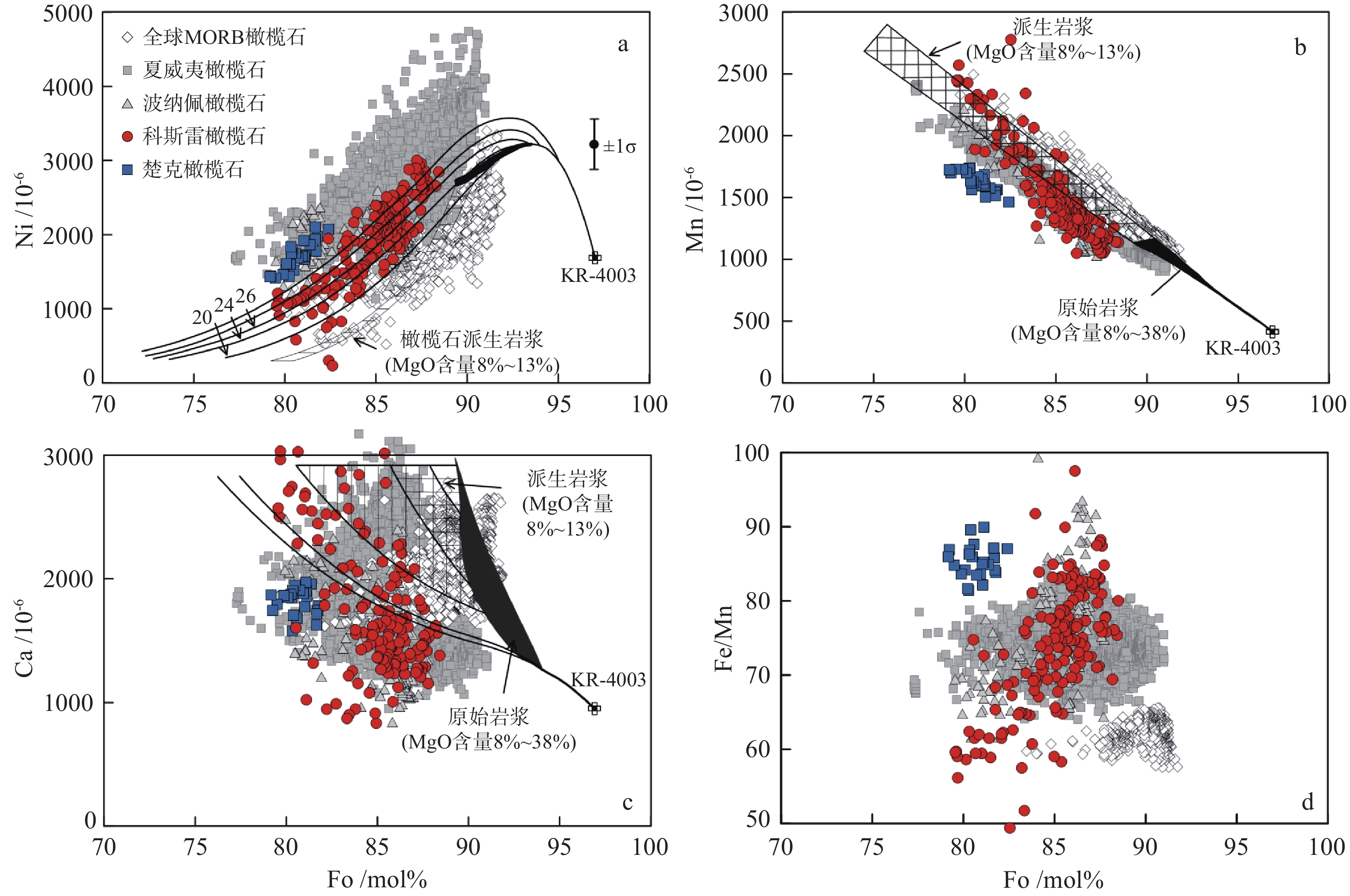
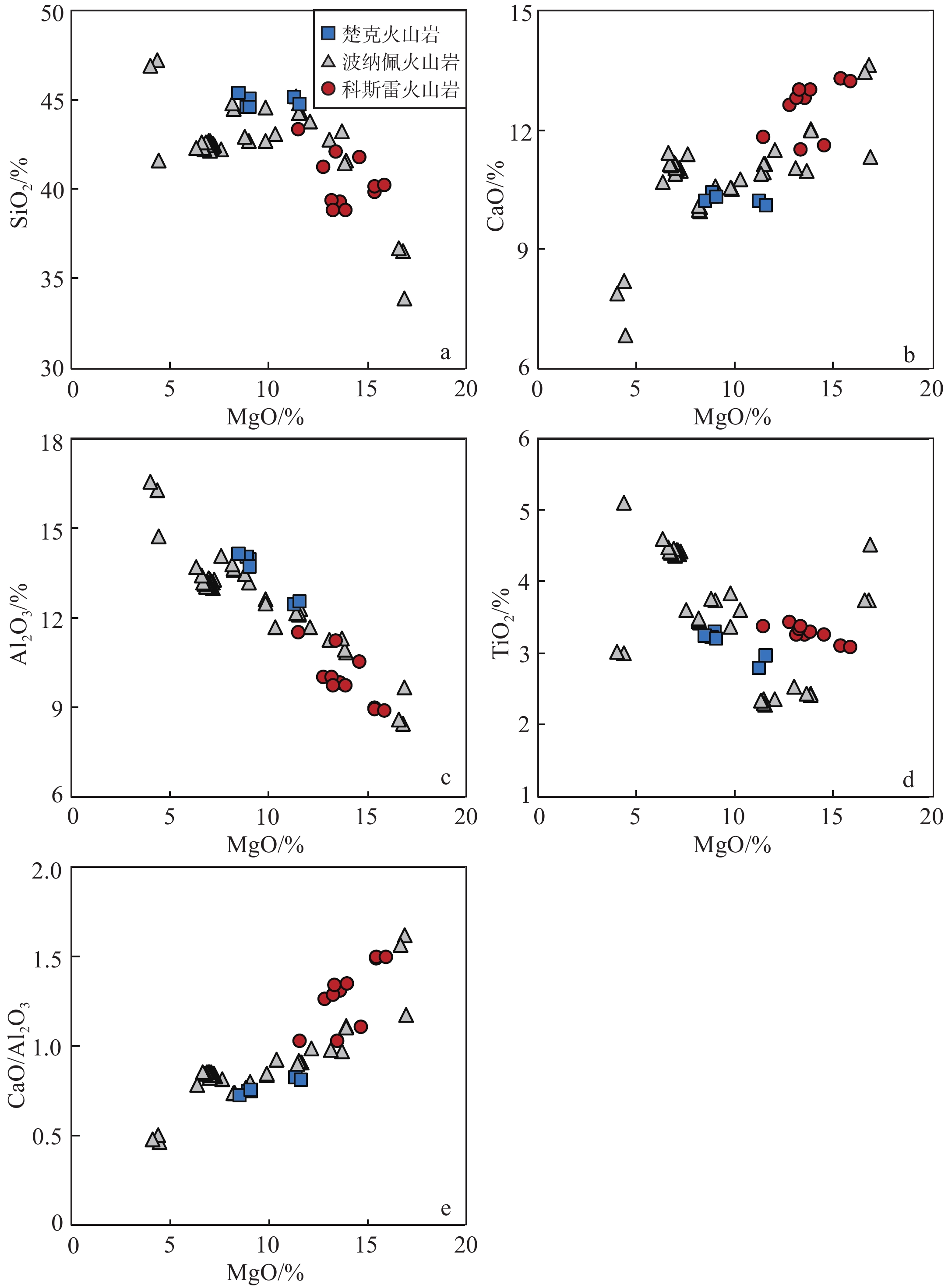
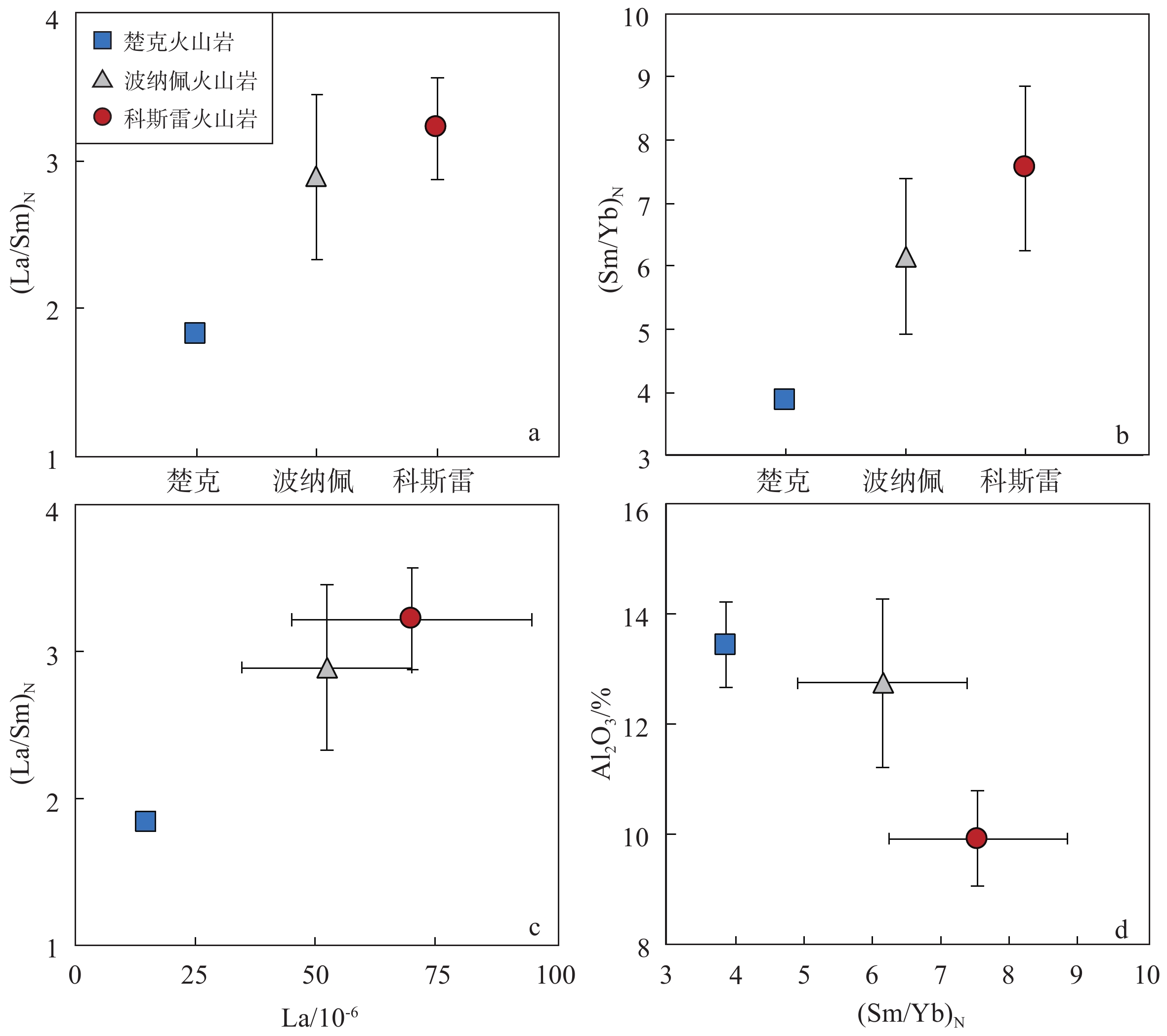
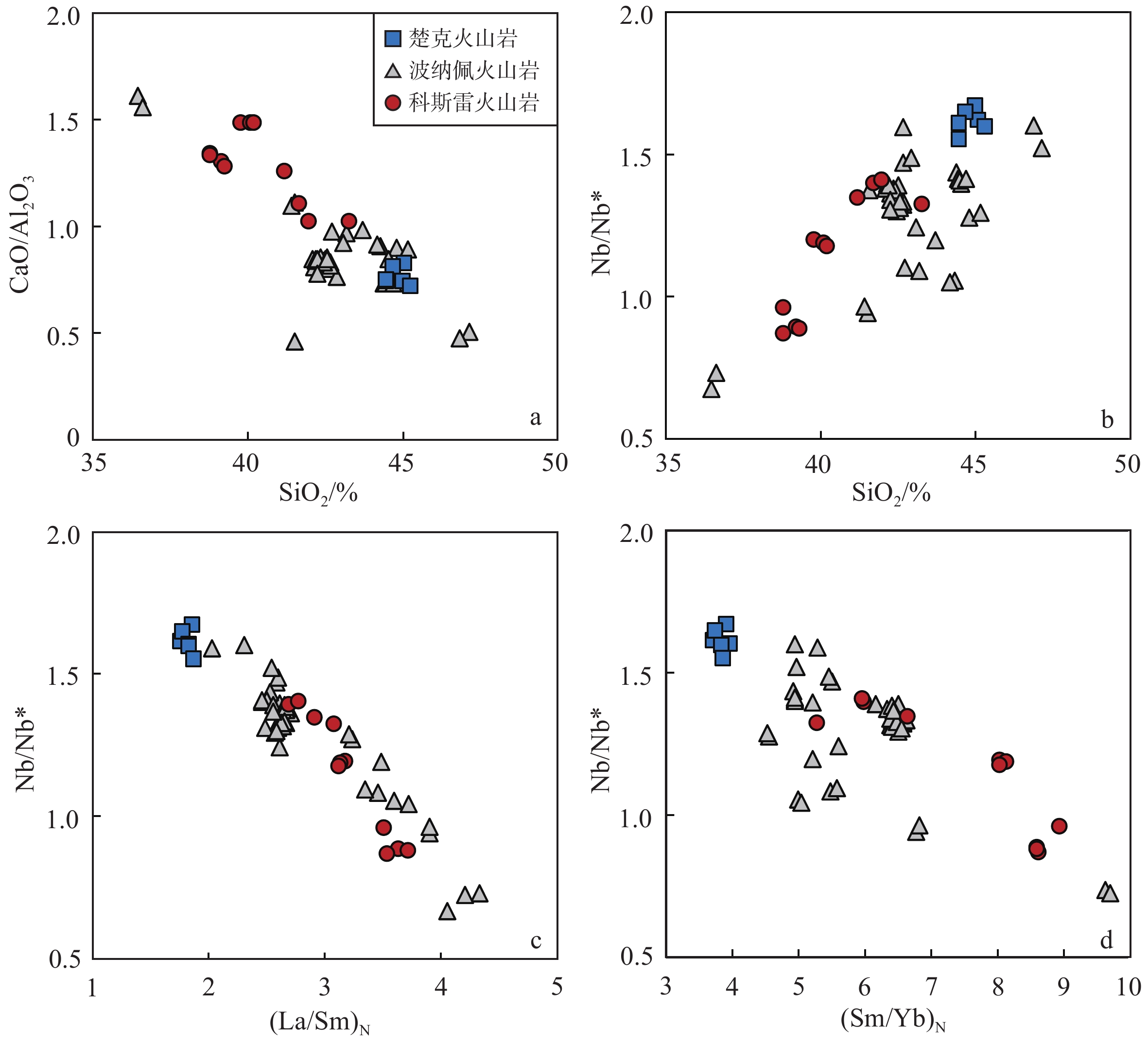
地幔柱活动不同阶段具有不同的岩浆作用产物,由楚克(14.8~4.3 Ma)、波纳佩(8.7~<1 Ma)、科斯雷(2~1 Ma)等洋岛构成的卡洛琳海山链是地幔柱活动不同阶段的典型例子,研究该海山链中不同洋岛地球化学特征的差异有助于加深对地幔柱晚期活动规律的认识。本研究对获取的楚克、科斯雷样品进行全岩主量、微量元素及矿物电子探针分析,并与波纳佩岛进行对比。科斯雷和楚克由霞石岩和碱性玄武岩组成,在微量元素配分模式上体现出典型碱性洋岛玄武岩的特征。这些样品的橄榄石斑晶具有与辉石岩源区夏威夷OIB橄榄石类似的高Ni、低Ca-Mn的特征,反映其地幔源区可能存在辉石岩。科斯雷霞石岩橄榄石斑晶内存在含碳酸盐的熔体包裹体,反映CO2在地幔熔融和岩浆成因过程中起到了重要作用。从楚克、波纳佩到科斯雷,La/Sm比值逐渐增大,地幔熔融程度逐渐降低。Nb/Nb*随着La/Sm、Sm/Yb升高和SiO2降低有逐渐降低的趋势,与地幔熔融程度降低过程中CO2作用的增强有关。研究认为,楚克、波纳佩、科斯雷等洋岛火山岩的地球化学变化由卡洛琳地幔柱热点活动逐渐减弱导致,随着地幔柱活动性减弱,CO2在火山岩成因上起到越来越明显的作用。
Abstract:The Caroline seamount chain consists of Chuuk (14.8~4.3 Ma), Pohnpei (8.7~<1 Ma), Kosrae (2~1 Ma) islands and a series of seamounts as the result of late-stage mantle plume. Geochemical variations in the seamount chain can deepen the understanding of late activity of the mantle plume. The whole rock major- and trace-elements, electron probe mineral analyses of the samples from Chuuk and Kosrae islands were conducted, and the results were compared with published data of Pohnpei. Kosrae and Chuuk islands are composed of nephelinites and alkaline basalts, reflecting typical ocean-island alkaline basalts in trace element patterns. Olivine phenocrysts in the samples are Ni-enriched but Ca-Mn–depleted, which is similar to olivines from Hawaiian OIB (ocean island basalt), suggesting the existence of pyroxenite in the mantle source. The presence of carbonate melt inclusions in the olivine phenocryst (Fo=85 mol%) of Kosrae nephelinite indicates that CO2 plays an important role in mantle melting and magma generation. The average La/Sm ratio of volcanic rocks gradually increases from Chuuk, Ponape, to Kosrae, which may reflect the decreasing degree of mantle melting during the weakening of the Caroline hot spot activity. In addition, the Nb/Nb* ratio decreases with the increase of La/Sm, Sm/Yb ratios and the decrease of SiO2, indicating the enhancing effect of CO2 due to the decrease in mantle melting degree. Therefore, the continuous geochemical changes of volcanic rocks from Chuuk, Ponape, Kosrae islands are caused by the gradual weakening of Caroline mantle plume activity, during which CO2 plays an increasingly obvious role in genesis of volcanic rocks.
-
Key words:
- mantle source /
- alkali basalt /
- degree of partial melting /
- olivine /
- Caroline seamount chain
-

-
表 1 楚克、科斯雷样品主量元素测定结果
Table 1. Whole-rock major element compositions of Chuuk and Kosrae samples
样品号 Na2O MgO Al2O3 SiO2 P2O5 K2O CaO TiO2 MnO Fe2O3T LOI 总计 CHK-1 2.16 11.30 12.40 45.10 0.31 0.57 10.20 2.79 0.16 14.50 − 99.49 CHK-4 2.44 9.04 13.90 45.00 0.36 0.76 10.30 3.28 0.17 14.90 0.38 100.52 CHK-5 2.20 11.60 12.50 44.70 0.31 0.59 10.10 2.96 0.16 15.00 0.49 100.61 CHK-6 2.24 8.90 14.00 44.50 0.36 0.68 10.40 3.22 0.16 14.20 1.36 100.01 CHK-7 2.47 8.53 14.10 45.30 0.38 0.67 10.20 3.24 0.16 14.10 1.38 100.52 CHK-8 2.35 9.07 13.70 44.50 0.37 0.74 10.30 3.19 0.17 14.60 0.84 99.82 KSR2-2 2.37 12.80 10.00 41.20 0.67 0.70 12.60 3.43 0.19 13.60 1.44 99.00 KSR4-1 4.00 13.60 9.79 39.20 1.20 0.97 12.80 3.25 0.21 13.80 0.24 99.06 KSR4-2 3.68 13.90 9.69 38.80 1.17 0.91 13.00 3.28 0.20 14.00 0.50 99.14 KSR4-4 4.06 13.20 9.98 39.30 1.21 0.93 12.80 3.26 0.21 13.80 0.37 99.12 KSR5-2 3.91 13.30 9.73 38.80 1.30 1.28 13.00 3.33 0.21 14.10 0.22 99.17 KSR6-1 0.71 15.40 8.97 39.80 1.04 0.10 13.30 3.09 0.19 13.60 2.93 99.13 KSR6-2 1.13 15.40 8.93 40.10 0.98 0.25 13.30 3.10 0.19 13.60 2.00 98.98 KSR6-3 0.91 15.90 8.86 40.20 0.99 0.17 13.20 3.08 0.19 13.70 2.34 99.54 KSR7-1 1.33 14.60 10.50 41.70 0.60 0.57 11.60 3.25 0.20 13.50 1.60 99.45 KSR7-2 1.74 13.40 11.20 42.00 0.66 0.75 11.50 3.36 0.17 13.50 1.52 99.81 KSR8-2 2.30 11.50 11.50 43.30 0.63 1.10 11.80 3.37 0.18 13.20 0.88 99.76 注:元素含量单位:%; CHK、KSR分别表示样品来自楚克、科斯雷;CHK-1因样品量太少而未做烧失量分析。 表 2 楚克、科斯雷样品微量元素测定结果
Table 2. Trace element compositions of Chuuk and Kosrae samples
10−6 样品号 Li Be Sc V Cr Co Ni Cu Zn Ga Rb Sr Y Zr Nb Cd Cs Ba La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Pb Th U CHK-1 4.02 0.89 27.5 327 537 70.0 389 130 105 20.1 11.2 383 21.7 139 19.9 0.236 0.145 163 13.5 30.4 4.07 18.5 4.98 1.68 5.13 0.81 4.45 0.83 1.99 0.261 1.48 0.211 3.59 1.28 0.165 1.02 1.29 0.338 CHK-4 4.72 1.03 25.8 374 286 63.2 247 98.5 115 20.5 15.9 572 23.3 161 24.0 0.242 0.091 204 16.0 35.6 4.72 21.2 5.54 1.88 5.66 0.88 4.81 0.88 2.13 0.276 1.56 0.218 4.01 1.52 0.097 1.13 1.49 0.416 CHK-5 4.33 0.86 26.5 345 401 69.6 363 124 116 19.1 11.1 490 21.2 142 20.6 0.233 0.097 170 13.6 32.3 4.07 18.5 4.95 1.68 5.11 0.81 4.36 0.81 1.96 0.258 1.46 0.207 3.69 1.34 0.150 1.03 1.32 0.361 CHK-6 4.52 1.03 26.7 367 308 58.9 226 104 115 21.1 14.6 595 22.8 157 22.8 0.246 0.253 192 15.7 35.5 4.65 20.9 5.53 1.87 5.64 0.87 4.70 0.87 2.09 0.272 1.54 0.215 3.96 1.47 0.072 1.11 1.48 0.408 CHK-7 52.0 0.97 25.9 365 258 68.0 265 107 123 21.5 8.45 584 24.2 164 23.8 0.288 0.069 208 16.3 37.3 4.84 21.8 5.75 1.95 5.89 0.91 4.92 0.91 2.21 0.286 1.65 0.229 4.12 1.52 0.066 1.43 1.55 0.442 CHK-8 4.64 0.91 24.1 330 273 55.8 226 90.5 105 18.5 16.6 483 22.5 147 21.5 0.230 0.125 184 15.3 33.4 4.51 20.3 5.30 1.81 5.47 0.85 4.59 0.86 2.06 0.264 1.52 0.210 3.68 1.33 0.056 1.08 1.43 0.377 KSR2-2 5.67 1.80 34.8 374 719 68.3 365 85.1 119 20.9 27.2 726 28.7 296 61.9 0.487 0.946 532 49.1 100 12.9 52.7 10.9 3.18 10.2 1.32 6.31 1.10 2.59 0.323 1.81 0.251 7.12 3.68 0.661 3.62 4.93 1.25 KSR4-1 12.2 2.92 29.9 404 686 79.8 445 95.4 155 28.4 31.2 1617 47.2 404 98.0 0.586 0.827 834 113 224 26.9 104 20.0 5.81 18.9 2.31 10.5 1.76 3.97 0.477 2.58 0.354 8.75 5.11 2.33 6.63 12.4 2.91 KSR4-2 10.3 2.28 25.8 348 603 69.6 392 78.5 134 23.9 21.5 1286 38.9 337 75.7 0.480 0.556 647 90.7 182 22.1 85.7 16.5 4.78 15.5 1.89 8.72 1.47 3.29 0.392 2.13 0.292 7.43 4.01 2.01 6.12 9.57 2.33 KSR4-4 10.9 2.49 24.7 347 566 67.1 372 71.3 134 24.1 30.3 1410 40.6 343 85.2 0.478 0.777 743 98.9 195 23.3 89.1 17.2 4.96 16.1 1.97 9.01 1.51 3.44 0.413 2.21 0.305 7.35 4.45 2.04 6.78 10.8 2.50 KSR5-2 10.6 2.58 25.6 355 573 68.4 358 74.1 143 24.5 30.7 1349 40.4 362 88.9 0.570 0.434 725 94.7 191 23.1 89.7 17.4 5.05 16.3 2.00 9.10 1.51 3.38 0.399 2.16 0.292 7.74 4.68 1.72 6.59 10.4 1.66 KSR6-1 10.3 1.84 28.8 265 918 70.8 455 82.9 111 20.5 56.2 1144 32.1 301 71.8 0.407 4.23 591 65.4 133 16.6 66.5 13.3 3.90 12.3 1.55 7.18 1.22 2.76 0.332 1.83 0.258 6.89 4.19 0.708 0.81 6.32 1.69 KSR6-2 6.94 1.82 29.4 310 933 71.2 457 79.1 111 20.4 66.9 1006 31.4 296 70.5 0.438 2.96 555 64.3 131 16.5 66.3 13.2 3.86 12.1 1.52 7.05 1.20 2.73 0.329 1.80 0.251 6.77 4.15 0.934 1.32 6.27 1.70 KSR6-3 7.74 1.87 28.9 276 949 71.7 463 77.7 113 20.3 53.2 1084 31.5 297 70.6 0.385 1.78 544 64.4 132 16.5 66.4 13.3 3.89 12.2 1.53 7.15 1.21 2.77 0.335 1.83 0.256 6.89 4.19 0.919 0.48 6.39 1.76 KSR7-1 5.56 1.64 31.0 348 791 71.0 438 85.3 110 20.2 14.8 432 29.1 260 53.4 0.356 0.311 540 40.3 82.2 10.6 44.1 9.64 2.89 8.96 1.22 6.07 1.08 2.54 0.320 1.78 0.248 6.30 3.19 0.635 2.43 4.16 1.05 KSR7-2 7.28 1.77 30.2 363 749 66.9 410 92.6 122 21.6 17.8 504 30.4 278 59.3 0.361 0.466 418 44.0 89.5 11.5 47.4 10.2 3.07 9.59 1.29 6.46 1.15 2.70 0.341 1.90 0.266 6.64 3.48 0.732 2.93 4.63 1.17 KSR8-2 5.74 1.83 30.7 332 511 58.1 285 84.0 109 20.6 30.6 684 31.2 296 62.6 0.449 0.699 513 48.5 98.1 12.4 49.7 10.2 3.01 9.64 1.31 6.55 1.19 2.88 0.371 2.13 0.304 6.97 3.74 0.678 3.22 5.28 1.36 -
[1] Ruttor S, Nebel O, Nebel-Yacobsen Y, et al. Alkalinity of ocean island lavas decoupled from enriched source components: a case study from the EM1-PREMA Tasmantid mantle plume [J]. Geochimica et Cosmochimica Acta, 2021, 314: 140-158. doi: 10.1016/j.gca.2021.09.023
[2] Garcia M O, Jorgenson B A, Mahoney J J, et al. An evaluation of temporal geochemical evolution of Loihi Summit Lavas: results from Alvin submersible dives [J]. Journal of Geophysical Research:Solid Earth, 1993, 98(B1): 537-550. doi: 10.1029/92JB01707
[3] Garcia M O, Foss D J P, West H B, et al. Geochemical and isotopic evolution of Loihi Volcano, Hawaii [J]. Journal of Petrology, 1995, 36(6): 1647-1674.
[4] Naumann T R, Geist D J. Generation of alkalic basalt by crystal fractionation of tholeiitic magma [J]. Geology, 1999, 27(5): 423-426. doi: 10.1130/0091-7613(1999)027<0423:GOABBC>2.3.CO;2
[5] Hirose K. Partial melt compositions of carbonated peridotite at 3 GPa and role of CO2 in alkali-basalt magma generation [J]. Geophysical Research Letters, 1997, 24(22): 2837-2840. doi: 10.1029/97GL02956
[6] Dasgupta R, Hirschmann M M, Smith N D. Partial Melting experiments of peridotite + CO2 at 3 GPa and genesis of alkalic ocean island basalts [J]. Journal of Petrology, 2007, 48(11): 2093-2124. doi: 10.1093/petrology/egm053
[7] Gerbode C, Dasgupta R. Carbonate-fluxed melting of MORB-like pyroxenite at 2·9 GPa and genesis of HIMU ocean island basalts [J]. Journal of Petrology, 2010, 51(10): 2067-2088. doi: 10.1093/petrology/egq049
[8] Kiseeva E S, Yaxley G M, Hermann J, et al. An experimental study of carbonated eclogite at 3·5–5·5 GPa—implications for silicate and carbonate metasomatism in the cratonic mantle [J]. Journal of Petrology, 2012, 53(4): 727-759. doi: 10.1093/petrology/egr078
[9] Kiseeva E S, Litasov K D, Yaxley G M, et al. Melting and phase relations of carbonated eclogite at 9–21 GPa and the petrogenesis of alkali-rich melts in the deep mantle [J]. Journal of Petrology, 2013, 54(8): 1555-1583. doi: 10.1093/petrology/egt023
[10] Mallik A, Dasgupta R. Reactive infiltration of MORB-eclogite-derived carbonated silicate melt into fertile peridotite at 3 GPa and genesis of alkalic magmas [J]. Journal of Petrology, 2013, 54(11): 2267-2300. doi: 10.1093/petrology/egt047
[11] Mallik A, Dasgupta R. Effect of variable CO2 on eclogite-derived andesite and lherzolite reaction at 3 GPa-Implications for mantle source characteristics of alkalic ocean island basalts [J]. Geochemistry, Geophysics, Geosystems, 2014, 15(4): 1533-1557. doi: 10.1002/2014GC005251
[12] Zhang G L, Chen L H, Jackson M G, et al. Evolution of carbonated melt to alkali basalt in the South China Sea [J]. Nature Geoscience, 2017, 10(3): 229-235. doi: 10.1038/ngeo2877
[13] Yao J H, Zhang G L, Wang S, et al. Recycling of carbon from the stagnant paleo-Pacific slab beneath Eastern China revealed by olivine geochemistry [J]. Lithos, 2021, 398-399: 106249. doi: 10.1016/j.lithos.2021.106249
[14] Jackson M G, Dasgupta R. Compositions of HIMU, EM1, and EM2 from global trends between radiogenic isotopes and major elements in ocean island basalts [J]. Earth and Planetary Science Letters, 2008, 276(1-2): 175-186. doi: 10.1016/j.jpgl.2008.09.023
[15] Jackson M G, Weis D, Huang S C. Major element variations in Hawaiian shield lavas: source features and perspectives from global ocean island basalt (OIB) systematics [J]. Geochemistry, Geophysics, Geosystems, 2012, 13(9): Q09009.
[16] Dasgupta R, Jackson M G, Lee C Y A. Major element chemistry of ocean island basalts — Conditions of mantle melting and heterogeneity of mantle source [J]. Earth and Planetary Science Letters, 2010, 289(3-4): 377-392. doi: 10.1016/j.jpgl.2009.11.027
[17] Mattey D P. The minor and trace element geochemistry of volcanic rocks from Truk, Ponape and Kusaie, Eastern Caroline Islands; the evolution of a young hot spot trace across old Pacific Ocean Crust [J]. Contributions to Mineralogy and Petrology, 1982, 80(1): 1-13. doi: 10.1007/BF00376730
[18] Keating B H, Mattey D P, Naughton J, et al. Age and origin of Truk Atoll, eastern Caroline Islands: geochemical, radiometric-age, and paleomagnetic evidence [J]. GSA Bulletin, 1984, 95(3): 350-356. doi: 10.1130/0016-7606(1984)95<350:AAOOTA>2.0.CO;2
[19] Keating B H, Mattey D P, Helsley C E, et al. Evidence for a hot spot origin of the Caroline Islands [J]. Journal of Geophysical Research:Solid Earth, 1984, 89(B12): 9937-9948. doi: 10.1029/JB089iB12p09937
[20] Jackson M G, Price A A, Blichert-Toft J, et al. Geochemistry of lavas from the Caroline hotspot, Micronesia: evidence for primitive and recycled components in the mantle sources of lavas with moderately elevated 3He/4He [J]. Chemical Geology, 2017, 455: 385-400. doi: 10.1016/j.chemgeo.2016.10.038
[21] Zhang G L, Zhang J, Wang S, et al. Geochemical and chronological constraints on the mantle plume origin of the Caroline Plateau [J]. Chemical Geology, 2020, 540: 119566. doi: 10.1016/j.chemgeo.2020.119566
[22] Zhang G L, Wang S, Zhang J, et al. Evidence for the essential role of CO2 in the volcanism of the waning Caroline mantle plume [J]. Geochimica et Cosmochimica Acta, 2020, 290: 391-407. doi: 10.1016/j.gca.2020.09.018
[23] Batanova V G, Thompson J M, Danyushevsky L V, et al. New olivine reference material for in situ microanalysis [J]. Geostandards and Geoanalytical Research, 2019, 43(3): 453-473. doi: 10.1111/ggr.12266
[24] Dixon T H, Batiza R, Futa K, et al. Petrochemistry, age and isotopic composition of alkali basalts from Ponape Island, Western Pacific [J]. Chemical Geology, 1984, 43(1-2): 1-28. doi: 10.1016/0009-2541(84)90138-4
[25] McDonough W F, Sun S S. The composition of the Earth [J]. Chemical Geology, 1995, 120(3-4): 223-253. doi: 10.1016/0009-2541(94)00140-4
[26] Hoernle K, Tilton G, Bas M J L, et al. Geochemistry of oceanic carbonatites compared with continental carbonatites: mantle recycling of oceanic crustal carbonate [J]. Contributions to Mineralogy and Petrology, 2002, 142(5): 520-542. doi: 10.1007/s004100100308
[27] Sobolev A V, Hofmann A W, Sobolev S V, et al. An olivine-free mantle source of Hawaiian shield basalts [J]. Nature, 2005, 434(7033): 590-597. doi: 10.1038/nature03411
[28] Sobolev A V, Hofmann A W, Kuzmin D V, et al. The amount of recycled crust in sources of mantle-derived melts [J]. Science, 2007, 316(5823): 412-417. doi: 10.1126/science.1138113
[29] Herzberg C. Identification of source lithology in the Hawaiian and Canary Islands: implications for origins [J]. Journal of Petrology, 2011, 52(1): 113-146. doi: 10.1093/petrology/egq075
[30] Prytulak J, Elliott T. TiO2 enrichment in ocean island basalts [J]. Earth and Planetary Science Letters, 2007, 263(3-4): 388-403. doi: 10.1016/j.jpgl.2007.09.015
[31] Garapić G, Mallik A, Dasgupta R, et al. Oceanic lavas sampling the high-3He/4He mantle reservoir: primitive, depleted, or re-enriched? [J]. American Mineralogist, 2015, 100(10): 2066-2081. doi: 10.2138/am-2015-5154
[32] Dasgupta R, Hirschmann M M, Stalker K. Immiscible Transition from carbonate-rich to silicate-rich melts in the 3 GPa melting interval of eclogite + CO2 and genesis of silica-undersaturated ocean island lavas [J]. Journal of Petrology, 2006, 47(4): 647-671. doi: 10.1093/petrology/egi088
[33] Spandler C, Yaxley G, Green D H, et al. Phase relations and melting of anhydrous K-bearing eclogite from 1200 to 1600°C and 3 to 5 GPa [J]. Journal of Petrology, 2008, 49(4): 771-795.
[34] Herzberg C. Petrology and thermal structure of the Hawaiian plume from Mauna Kea volcano [J]. Nature, 2006, 444(7119): 605-609. doi: 10.1038/nature05254
[35] Kogiso T, Hirschmann M M, Frost D J. High-pressure partial melting of garnet pyroxenite: possible mafic lithologies in the source of ocean island basalts [J]. Earth and Planetary Science Letters, 2003, 216(4): 603-617. doi: 10.1016/S0012-821X(03)00538-7
[36] Kogiso T, Hirschmann M M. Partial melting experiments of bimineralic eclogite and the role of recycled mafic oceanic crust in the genesis of ocean island basalts [J]. Earth and Planetary Science Letters, 2006, 249(3-4): 188-199. doi: 10.1016/j.jpgl.2006.07.016
[37] Andersen T, Neumann E R. Fluid inclusions in mantle xenoliths [J]. Lithos, 2001, 55(1-4): 301-320. doi: 10.1016/S0024-4937(00)00049-9
[38] Golovin A V, Sharygin V V, Pokhilenko N P. Melt inclusions in olivine phenocrysts in unaltered kimberlites from the Udachnaya-East pipe, Yakutia: some aspects of kimberlite magma evolution during late crystallization stages [J]. Petrology, 2007, 15(2): 168-183. doi: 10.1134/S086959110702004X
[39] Frezzotti M L, Touret J L R. CO2, carbonate-rich melts, and brines in the mantle [J]. Geoscience Frontiers, 2014, 5(5): 697-710. doi: 10.1016/j.gsf.2014.03.014
[40] Hudgins T R, Mukasa S B, Simon A C, et al. Melt inclusion evidence for CO2-rich melts beneath the western branch of the East African Rift: implications for long-term storage of volatiles in the deep lithospheric mantle [J]. Contributions to Mineralogy and Petrology, 2015, 169(5): 46. doi: 10.1007/s00410-015-1140-9
-




 下载:
下载: