Variations in the length and weight of Noelaerhabdaceae coccolith in the Late Quaternary tropical Western Pacific and their influencing factors
-
摘要:
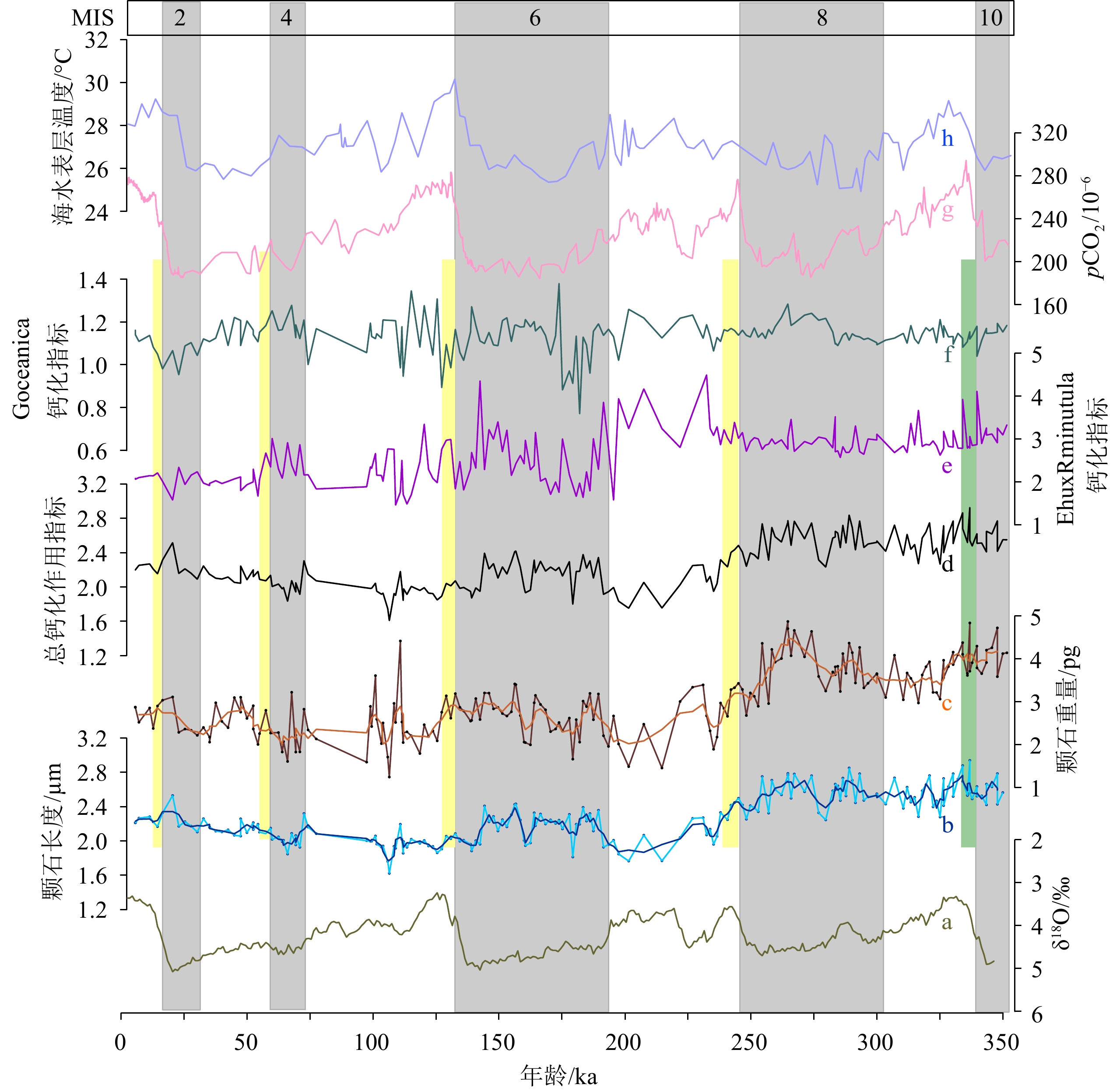
选取热带西太平洋暖池北部边缘西菲律宾海本哈姆高原(Benham Rise)MD06-3050站位的柱状样沉积物样品,利用双向圆偏光法拍摄并合成图像,通过SYRACO人工智能软件自动识别并测量和计算Noelaerhabdaceae科颗石的平均长度和平均质量。研究结果显示,自35万年以来,Noelaerhabdaceae科平均颗石长度和质量的变化趋势具有较强的相似性,两者的冰期/间冰期变化特征均不明显。根据颗石长度和质量计算得到的颗石钙化作用指标曲线与前两者的变化趋势也存在一定的相似性,说明研究海区颗石的平均质量和长度均可作为指示颗石藻钙化作用程度的指标。通过与冰芯记录的大气pCO2变化曲线进行对比,发现在大气pCO2浓度较高的冰期终止期以及间冰期早期,该科颗石的钙化作用相对较低,表明pCO2在一定程度上影响了颗石藻的钙化作用。此外,对颗石的平均长度和质量进行23 ka周期滤波,两滤波曲线的变化与北纬15°夏季平均日照辐射曲线的变化较为一致,指示了地球天文轨道参数对颗石藻演化的调控作用。
Abstract:Sediment samples from core MD06-3050 in the Benham Rise in the Western Philippine Sea at the northern edge of the tropical Western Pacific Warm Pool were selected for Family Noelaerhabdaceae coccolith size analyses. Images were taken and synthesized using bidirectional circular polarization method. The average coccolith length and weight were automatically identified and measured by SYRACO artificial intelligence software. Results show that the average coccolith length and weight show strong similarities in the last 350000 years, with no obvious glacial-interglacial changes. The coccolith calcification index was estimated based on the length and weight of coccolith, and shows the same patterns of the morphological parameters, but presents a more obvious glacial-interglacial pattern. The coccolith calcification index was compared with the ice-core atmospheric pCO2, which shows that the calcification of Noelaerhabdaceae coccoliths was weakened in the glaciation termination period and the early-middle interglacial periods when the atmospheric pCO2 concentration was higher. The 23-ka filtering of the average coccolith length and weight agrees with the summer average solar radiation at 15°N, suggesting the regulation of the Earth's astronomical orbit parameters on the evolution of coccolithophores.
-
Key words:
- coccolith weight /
- coccolith length /
- Late Quaternary /
- Western Pacific
-

-
表 1 Noelaerhabdaceae 科颗石长度和质量的平均值和标准偏差
Table 1. Average and standard deviation of Noelaerhabdaceae coccolith length and mass
颗石类别 EhuxRminutula Rhaqii Gericsonii Gcaribbeanica Goceanica Povata 长度平均值/μm 2.5 2.15 1.94 3.00 3.68 3.91 平均长度的标准偏差/μm 0.81 0.81 0.55 0.70 0.43 0.61 质量平均值/pg 3.01 2.90 1.76 5.64 6.19 7.11 平均质量的标准偏差/pg 1.83 1.98 1.30 2.27 1.74 1.85 -
[1] Sabine C L, Feely R A, Gruber N, et al. The Oceanic Sink for Anthropogenic CO2[J]. Science, 2004, 305(5682): 367-371. doi: 10.1126/science.1097403
[2] Fabry V J, Seibel B A, Feely R A, et al. Impacts of ocean acidification on marine fauna and ecosystem processes [J]. ICES Journal of Marine Science, 2008, 65(3): 414-432. doi: 10.1093/icesjms/fsn048
[3] Rost B, Riebesell U. Coccolithophores and the biological pump: responses to environmental changes[M]//Thierstein H R, Young J R. Coccolithophores: From Molecular Processes to Global Impact. Berlin: Springer, 2004: 99-125.
[4] Brownlee C, Taylor A. Calcification in coccolithophores: a cellular perspective[M]//Thierstein H R, Young J R. Coccolithophores: From Molecular Processes to Global Impact. Berlin: Springer, 2004: 31-49.
[5] Milliman J D. Production and accumulation of calcium carbonate in the ocean: budget of a nonsteady state[J]. Global Biogeochemical Cycles, 1993, 7(4): 927-957. doi: 10.1029/93GB02524
[6] Beaufort L. Weight estimates of coccoliths using the optical properties (birefringence) of calcite[J]. Micropaleontology, 2005, 51(4): 289-297. doi: 10.2113/gsmicropal.51.4.289
[7] Beaufort L, Barbarin N, Gally Y. Optical measurements to determine the thickness of calcite crystals and the mass of thin carbonate particles such as coccoliths[J]. Nature Protocols, 2014, 9(3): 633-642. doi: 10.1038/nprot.2014.028
[8] Beaufort L, Bolton C T, Sarr A- C, et al. Cyclic evolution of phytoplankton forced by changes in tropical seasonality[J]. Nature, 2022, 601(7891): 79-84. doi: 10.1038/s41586-021-04195-7
[9] Sun H J, Li T G, Chang F M, et al. Deep-sea carbonate preservation in the western Philippine Sea over the past 1Ma[J]. Quaternary International, 2017, 459: 101-115. doi: 10.1016/j.quaint.2017.08.041
[10] Sun H J, Li T G, Liu C L, et al. Variations in the western Pacific warm pool across the mid-Pleistocene: Evidence from oxygen isotopes and coccoliths in the West Philippine Sea[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2017, 483: 157-171. doi: 10.1016/j.palaeo.2017.07.008
[11] Lisiecki L E, Raymo M E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records[J]. Paleoceanography, 2005, 20(1): PA1003.
[12] Beaufort L. Adaptation of the random settling method for quantitative studies of calcareous nannofossils[J]. Micropaleontology, 1991, 37(4): 415-418. doi: 10.2307/1485914
[13] Beaufort L, Dollfus D. Automatic recognition of coccoliths by dynamical neural networks[J]. Marine Micropaleontology, 2004, 51(1-2): 57-73. doi: 10.1016/j.marmicro.2003.09.003
[14] Barbarin N. La Reconnaissance Automatisée des Nannofossiles Calcaires du Cénozoique[D]. Français: Aix-Marseille University, 2014.
[15] Young J R. Neogene[M]//Bown P R. Calcareous Nannofossil Biostratigraphy. London: Micropalaeontological Society Publication Series, 1998.
[16] Jin X B, Ma W T, Liu C L. Origin of the long-term increase in coccolith size and its implication for carbon cycle and climate over the past 2 Myr[J]. Quaternary Science Reviews, 2022, 290: 107642. doi: 10.1016/j.quascirev.2022.107642
[17] Su X, Liu C L, Beaufort L. Late Quaternary coccolith weight variations in the northern South China Sea and their environmental controls[J]. Marine Micropaleontology, 2020, 154: 101798. doi: 10.1016/j.marmicro.2019.101798
[18] D’Amario B, Ziveri P, Grelaud M, et al. Emiliania huxleyi coccolith calcite mass modulation by morphological changes and ecology in the Mediterranean Sea[J]. PLoS One, 2018, 13(7): e0201161. doi: 10.1371/journal.pone.0201161
[19] Young J R, Ziveri P. Calculation of coccolith volume and it use in calibration of carbonate flux estimates[J]. Deep Sea Research II: Tropical Studies in Oceanography, 2000, 47(9-11): 1679-1700.
[20] Monnin E, Indermühle A, Dällenbach A, et al. Atmospheric CO2 concentrations over the last glacial termination[J]. Science, 2001, 291(5501): 112-114. doi: 10.1126/science.291.5501.112
[21] Petit J R, Jouzel J, Raynaud D, et al. Climate and atmospheric history of the past 420, 000 years from the Vostok ice core, Antarctica[J]. Nature, 1999, 399(6735): 429-436. doi: 10.1038/20859
[22] Jia Q, Li T G, Xiong Z F, et al. Hydrological variability in the western tropical Pacific over the past 700 kyr and its linkage to Northern Hemisphere climatic change[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2018, 493: 44-54. doi: 10.1016/j.palaeo.2017.12.039
[23] Jaccard S L, Galbraith E D, Sigman D M, et al. Subarctic Pacific evidence for a glacial deepening of the oceanic respired carbon pool[J]. Earth and Planetary Science Letters, 2009, 277(1-2): 156-165. doi: 10.1016/j.jpgl.2008.10.017
[24] Beaufort L, Probert I, de Garidel-Thoron T, et al. Sensitivity of coccolithophores to carbonate chemistry and ocean acidification[J]. Nature, 2011, 476(7358): 80-83. doi: 10.1038/nature10295
[25] Bach L T, Bauke C, Meier K J S, et al. Influence of changing carbonate chemistry on morphology and weight of coccoliths formed by Emiliania huxleyi[J]. Biogeosciences, 2012, 9(8): 3449-3463. doi: 10.5194/bg-9-3449-2012
[26] De Bodt C, Van Oostende N, Harlay J, et al. Individual and interacting effects of pCO2 and temperature on Emiliania huxleyi calcification: study of the calcite production, the coccolith morphology and the coccosphere size[J]. Biogeosciences, 2010, 7(5): 1401-1412. doi: 10.5194/bg-7-1401-2010
[27] Zondervan I, Rost B, Riebesell U. Effect of CO2 concentration on the PIC/POC ratio in the coccolithophore Emiliania huxleyi grown under light-limiting conditions and different day-lengths[J]. Journal of Experimental Marine Biology and Ecology, 2002, 272(1): 55-70. doi: 10.1016/S0022-0981(02)00037-0
[28] Barker S, Archer D, Booth L, et al. Globally increased pelagic carbonate production during the Mid-Brunhes dissolution interval and the CO2 paradox of MIS 11[J]. Quaternary Science Reviews, 2006, 25(23-24): 3278-3293. doi: 10.1016/j.quascirev.2006.07.018
[29] Bollmann J, Baumann K H, Thierstein H R. Global dominance of Gephyrocapsa coccoliths in the late Pleistocene: selective dissolution, evolution, or global environmental change?[J]. Paleoceanography, 1998, 13(5): 517-529. doi: 10.1029/98PA00610
[30] Meier K J S, Berger C, Kinkel H. Increasing coccolith calcification during CO2 rise of the penultimate deglaciation (Termination II)[J]. Marine Micropaleontology, 2014, 112: 1-12. doi: 10.1016/j.marmicro.2014.07.001
[31] Molfino B, McIntyre A. Precessional forcing of nutricline dynamics in the Equatorial Atlantic[J]. Science, 1990, 249(4970): 766-769. doi: 10.1126/science.249.4970.766
[32] Beaufort L, Lancelot Y, Camberlin P, et al. Insolation cycles as a major control of equatorial Indian Ocean primary production[J]. Science, 1997, 278(5342): 1451-1454. doi: 10.1126/science.278.5342.1451
[33] Beaufort L, de Garidel-Thoron T, Mix A C, et al. ENSO-like forcing on oceanic primary production during the Late Pleistocene[J]. Science, 2001, 293(5539): 2440-2444. doi: 10.1126/science.293.5539.2440
[34] 孙晗杰, 李铁刚, 苏翔, 等. 中更新世以来西菲律宾海上层水体结构演化特征: 来自钙质超微化石 Florisphaera profunda的证据[J]. 第四纪研究, 2011, 31(2): 216-226
SUN Hanjie, LI Tiegang, SU Xiang, et al. Upper water mass structure evolution in the western Philippine Sea since mid-Pleistocene: evidence from the abundance of coccolith species Florisphaera profunda[J]. Quaternary Sciences, 2011, 31(2): 216-226.
[35] Li M S, Hinnov L, Kump L. Acycle: Time-series analysis software for paleoclimate research and education[J]. Computers & Geosciences, 2019, 127: 12-22.
[36] Laskar J, Robutel P, Joutel F, et al. A long-term numerical solution for the insolation quantities of the Earth[J]. Astronomy and Astrophysics, 2004, 428(1): 261-285. doi: 10.1051/0004-6361:20041335
[37] Cavaleiro C, Voelker A H L, Stoll H, et al. Insolation forcing of coccolithophore productivity in the North Atlantic during the Middle Pleistocene[J]. Quaternary Science Reviews, 2018, 191: 318-336. doi: 10.1016/j.quascirev.2018.05.027
[38] Berger A. Milankovitch theory and climate[J]. Reviews of Geophysics, 1988, 26: 624-657. doi: 10.1029/RG026i004p00624
[39] Valliina S M, Simó R. Strong relationship between DMS and the solar radiation dose over the global surface ocean[J]. Science, 2007, 315(5811): 506-508.
[40] Nimer N A, Merrett, M J. Calcification rate in Emiliania huxleyi Lohmann in response to light, nitrate and availability of inorganic carbon[J]. New Phytologist, 1993, 123(4): 673-677.
[41] Zhang Y. Effects of Temperature, Carbonate Chemistry and Light Intensity on the Coccolithophores Emiliania Huxleyi and Gephyrocapsa Oceanica[D]. University of Kiel, 2014.
[42] Zondervan, I. The effects of light, macronutrients, trace metals and CO2 on the production of calcium carbonate and organic carbon in coccolithophores-a review[J]. Deep-Sea Research II: Tropical Studies in Oceanography, 2007, 54(5-7): 521–537. doi: 10.1016/j.dsr2.2006.12.004
[43] van Bleijswijk J D L, Kempers R S, Veldhuis M J, et al. Cell and growth characteristics of type A and B of Emiliania huxleyi (Prymnesiophyceae) as determined by flow cytometry and chemical analyses[J]. Journal of Phycology, 1994, 30(2): 230–241. doi: 10.1111/j.0022-3646.1994.00230.x
-




 下载:
下载: