Orthogonal Experiments of Copper Slag Particles Direct Reduction by Carbon-Containing Solid Waste Reductant
-
摘要:
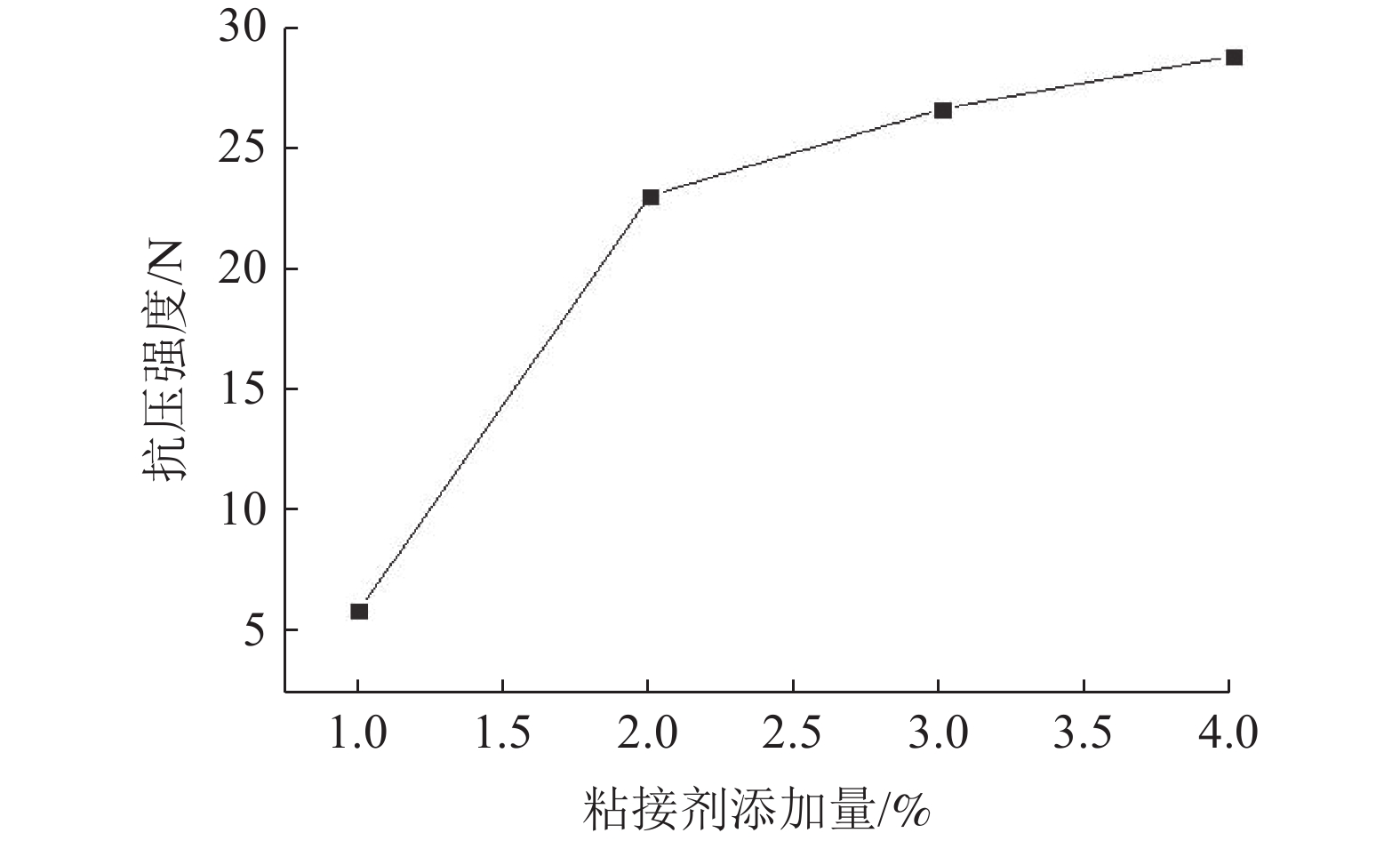
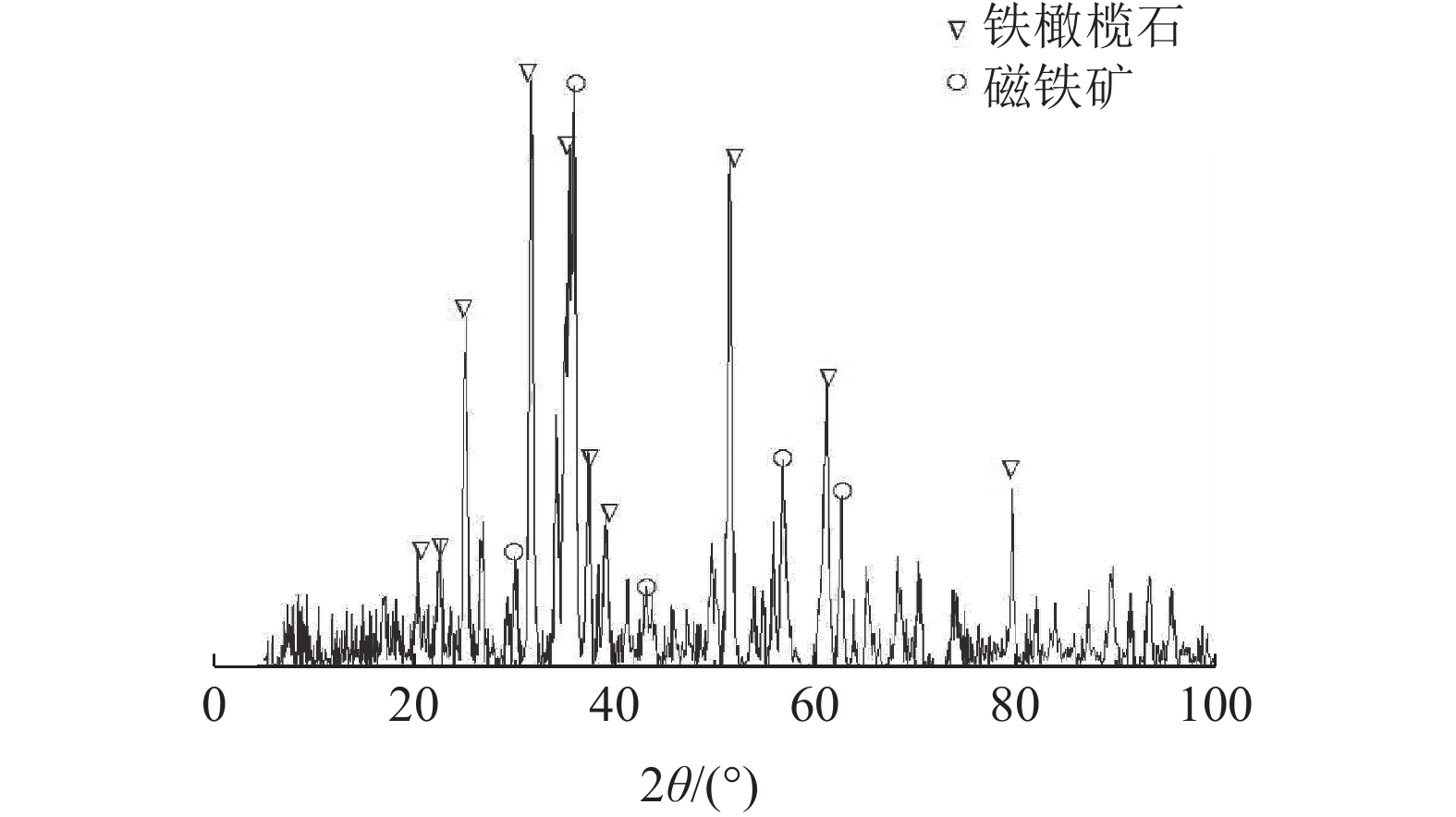
通过转杯离心粒化法制备铜渣颗粒。以铜渣颗粒、碳质还原剂、粘结剂和造渣剂为主要原料制备铜渣含碳球团,在实验条件下,六种考查因素对铜渣含碳球团还原率影响的主次关系为:反应温度>造渣剂配比>气氛>还原剂种类>铜渣粒径>还原剂配比。通过极差分析得出铜渣含碳球团直接还原较佳还原条件:反应温度为1150℃,造渣剂配比(S/CaO)为1∶0.4,实验气氛为CO2(50%)N2(50%),还原剂为煤粉,铜渣粒径为+0.425 mm,还原剂配比(C/O)为1.2∶1,此时铜渣的还原率为98.2%。
Abstract:Copper slag particles were prepared by rotary cup atomizer. Carbon-containing copper slag pellets were prepared by copper slag particles, carbon reductant, binder and slag former. The effects of six factors on reduction ratio of carbon-containing copper slag pellets were in accordance with the sequence of reaction temperature > the ratio of slag former > atmosphere > the types of reduction reductant > particle size of copper slag > the addition ratio of reductant, under experiment conditions. The optimum condition for direct reduction of carbon-containing copper slag pellets was that the reaction temperature was 1150℃, the ratio of slag former was 1∶0.4, atmosphere was CO2(50%)+N2(50%), the reductant was coal, particle size of copper slag was +0.425 mm and the addition ratio of reductant was 1.2∶1. On this condition, the reduction ratio of copper slag is 98.2%.
-
Key words:
- Copper slag /
- Centrifugation /
- Direct reduction
-

-
表 1 铜渣化学成分/%
Table 1. Chemical composition of copper slag
FeO Fe3O4 CaO Al2O3 MFe SiO2 Cu MgO S Zn 其他 37.50 18.90 0.23 0.98 1.24 31.99 0.74 0.42 0.39 2.78 4.87 表 2 还原剂工业分析/%
Table 2. Industrial analysis of reducing agents
还原剂种类 水分 挥发分 灰分 固定碳 煤粉 6.59 26.05 34.41 32.95 煤焦 0.22 1.47 28.61 69.7 生物质 3.93 77.31 3.13 15.63 生物质碳 0.44 6.76 18.07 74.73 塑料焦 0.11 0.92 8.6 90.37 表 3 实验工况
Table 3. Experimental conditions
考查因素 气氛(CO2/N2) 反应温度/℃ 铜渣粒径/mm 还原剂配比(C/O) 造渣剂配比(S/CaO) 还原剂种类 1 N2 800 -0.074 0.8∶1 1∶0 煤粉 2 CO2(25%)N2(75%) 900 -0.106+0.074 0.9∶1 1∶0.1 煤焦 3 CO2(50%)N2(50%) 1000 +0.71 1∶1 1∶0.2 生物质 4 CO2(75%)N2(25%) 1100 -0.71+0.425 1.1∶1 1∶0.3 生物质碳 5 CO2 1150 -0.425 1.2∶1 1∶0.4 塑料焦 表 4 铜渣含碳球团直接还原正交实验结果
Table 4. Orthogonal experimental results of direct reduction for carbon-bearing pellets of copper slag
序号 气氛(CO2N2) 反应温度/℃ 铜渣粒径/mm 还原剂配比(C/O) 造渣剂配比(S/CaO) 还原剂种类 还原率/% 1 N2 800 -0.074 0.8∶1 1∶0 煤粉 14.97 2 N2 900 -0.106+0.074 0.9∶1 1∶0.1 煤焦 29.08 3 N2 1000 +0.71 1∶1 1∶0.2 生物质 51.28 4 N2 1100 -0.71+0.425 1.1∶1 1∶0.3 生物质碳 80.91 5 N2 1150 -0.425 1.2∶1 1∶0.4 塑料焦 93.8 6 CO2(25%)N2(75%) 800 -0.106+0.074 1.1∶1 1∶0.3 塑料焦 67.17 7 CO2(25%)N2(75%) 900 +0.71 1.2∶1 1∶0.4 煤粉 73.7 8 CO2(25%)N2(75%) 1000 -0.71+0.425 0.8∶1 1∶0 煤焦 30.23 9 CO2(25%)N2(75%) 1100 -0.425 0.9∶1 1∶0.1 生物质 66.96 10 CO2(25%)N2(75%) 1150 -0.074 1∶1 1∶0.2 生物质碳 92.51 11 CO2(50%)N2(50%) 800 -0.106+0.074 0.9∶1 1∶0.1 生物质碳 77.6 12 CO2(50%)N2(50%) 900 +0.71 1∶1 1∶0.2 塑料焦 75.38 13 CO2(50%)N2(50%) 1000 -0.71+0.425 1.1∶1 1∶0.3 煤粉 87.31 14 CO2(50%)N2(50%) 1100 -0.074 1.2∶1 1∶0.4 煤焦 89.97 15 CO2(50%)N2(50%) 1150 -0.106+0.074 0.8∶1 1∶0 生物质 74.58 16 CO2(75%)N2(25%) 800 +0.71 1.2∶1 1∶0.4 生物质 69.34 17 CO2(75%)N2(25%) 900 -0.71+0.425 0.8∶1 1∶0 生物质碳 39.68 18 CO2(75%)N2(25%) 1000 -0.074 0.9∶1 1∶0.1 塑料焦 62.42 19 CO2(75%)N2(25%) 1100 -0.106+0.074 1∶1 1∶0.2 煤粉 86.34 20 CO2(75%)N2(25%) 1150 +0.71 1.1∶1 1∶0.3 煤焦 96.6 21 CO2 800 -0.71+0.425 1∶1 1∶0.2 煤焦 29.03 22 CO2 900 -0.074 1.1∶1 1∶0.3 生物质 28.98 23 CO2 1000 -0.106+0.074 1.2∶1 1∶0.4 生物质碳 88.3 24 CO2 1100 +0.71 0.8∶1 1∶0 塑料焦 67.19 25 CO2 1150 -0.71+0.425 0.9∶1 1∶0.1 煤粉 97.94 Ⅰ 270.04 258.11 288.91 226.65 226.65 360.27 Ⅱ 330.56 246.81 345.47 334.01 334.01 274.91 Ⅲ 404.83 319.55 366.38 334.53 334.53 291.14 Ⅳ 354.39 391.38 353.78 360.97 360.97 379.01 Ⅴ 311.45 455.43 316.79 415.12 415.12 365.95 Ⅰ/5 54.00 51.62 57.58 45.33 45.33 72.05 Ⅱ/5 66.11 49.36 69.09 66.8 66.8 54.98 Ⅲ/5 80.97 63.91 73.28 66.91 66.91 58.22 Ⅳ/5 70.88 78.28 70.76 72.19 72.19 75.8 Ⅴ/5 62.29 91.09 63.36 83.02 83.02 73.19 极差 26.96 41.72 15.50 37.69 37.69 20.82 -
[1] 姜平国, 吴朋飞, 胡晓军, 等. 铜渣综合利用研究现状及其新技术的提出[J]. 中国矿业, 2016, 25(2):76-79. doi: 10.3969/j.issn.1004-4051.2016.02.014
JIANG P G, WU P F, HU X J, et al. Research review of comprehensive utilization of copper slag and new technology[J]. China Mining Magazine, 2016, 25(2):76-79. doi: 10.3969/j.issn.1004-4051.2016.02.014
[2] 孙伟, 刘建远, 贺政, 等. 某铜渣浮选试验研究[J]. 矿产综合利用, 2019(2):112-114. doi: 10.3969/j.issn.1000-6532.2019.02.023
SUN W, LIU J Y, HE Z, et al. Study on flotation of copper slag[J]. Multipurpose Utilization of Mineral Resources, 2019(2):112-114. doi: 10.3969/j.issn.1000-6532.2019.02.023
[3] YANG Z H, LIN Q, XIA J X, et al. Preparation and crystallization of glass–ceramics derived from iron-rich copper slag [J]. Journal of Alloys and Compounds, 2013, 574: 354-360.
[4] LIU H Y, LU H X, CHEN D L, et al. Preparation and properties of glass–ceramics derived from blast-furnace slag by a ceramic-sintering process [J]. Ceramics International. 2009, 35(8): 3181-3184.
[5] Rudnik E, Burzńska L, Gumowska W. Hydrometallurgical recovery of copper and cobalt from reduction-roasted copper converter slag[J]. Minerals Engineering, 2009, 22(1):88-95. doi: 10.1016/j.mineng.2008.04.016
[6] 邓彤, 凌云汉. 含钴铜转炉渣的工艺矿物学[J]. 中国有色金属学报, 2001, 11(5):881-885. doi: 10.3321/j.issn:1004-0609.2001.05.029
DENG T, LING Y H. Process mineralogy of Cobalt - bearing copper converter slag[J]. Chinese Journal of Nonferrous Metals, 2001, 11(5):881-885. doi: 10.3321/j.issn:1004-0609.2001.05.029
[7] Gyurov Stoyko, Rabadjieva Diana, Kovacheva Daniela, et al. Kinetics of copper slag oxidation under nonisothermal conditions[J]. Journal of Thermal Analysis and Calorimetry, 2014, 116(2):945-954. doi: 10.1007/s10973-013-3569-2
[8] Siwiec G, Oleksiak B, Matula T, et al. Reduction of copper slag with the use of carbon granulates [J]. Metallurgy. 2014, 53(4): 585-587.
[9] 邓彤, 文震, 刘东. 硫酸介质中氯化物参与下氧化浸出铜渣过程[J]. 中国有色金属学报, 2001, 11(2):302-306. doi: 10.3321/j.issn:1004-0609.2001.02.030
DENG T, WEN Z, LIU D. Leaching of copper residue with oxygen in sulfuric acid with participation of chloride[J]. Chinese Journal of Nonferrous Metals, 2001, 11(2):302-306. doi: 10.3321/j.issn:1004-0609.2001.02.030
[10] Warczok A, Riveros G. Slag cleaning in crossed electric and magnetic fields[J]. Minerals Engineering. 2007, 20(1): 34-43.
[11] 马泳波, 杜雪岩, Alibek Kakimov, 等. 富铁镍渣综合利用的研究与进展综述[J]. 矿产综合利用, 2018(6):25-31. doi: 10.3969/j.issn.1000-6532.2018.06.005
MA Y B, DU X Y, Alibek Kakimov, et al. Research and progress of nickel slag's comprehensive utilization[J]. Multipurpose Utilization of Mineral Resources, 2018(6):25-31. doi: 10.3969/j.issn.1000-6532.2018.06.005
[12] 李伟. 云南某低品位含铁硫化铜矿综合回收试验研究[J]. 矿产综合利用, 2018(1):50-54. doi: 10.3969/j.issn.1000-6532.2018.01.011
LI W. Experimental study on comprehensive recovery for one low-grade copper sulphide ore containing iron in Yunnan[J]. Multipurpose Utilization of Mineral Resources, 2018(1):50-54. doi: 10.3969/j.issn.1000-6532.2018.01.011
[13] SUN Y S, HAO Y X, GAO P, et al. Thermogravimetric study of coal-based reduction of oolitic iron ore: Kinetics and mechanisms[J]. International Journal of Mineral Processing, 2015, 143:87-97. doi: 10.1016/j.minpro.2015.09.005
[14] 李涛, 刘晨, 佘世杰. 铜渣中铁铜回收的实验研究[J]. 矿产综合利用, 2020(2):145-150. doi: 10.3969/j.issn.1000-6532.2020.02.026
LI T, LIU C, SHE S J. Research on recovery of iron and copper in copper slag[J]. Multipurpose Utilization of Mineral Resources, 2020(2):145-150. doi: 10.3969/j.issn.1000-6532.2020.02.026
[15] 徐冬林, 谢冬冬, 张旭, 等. 基于均匀试验的赤铁矿石助磨剂复配试验研究[J]. 矿产综合利用, 2019(2):30-36. doi: 10.3969/j.issn.1000-6532.2019.02.006
XU D L, XIE D D, ZHANG X, et al. Experimental study on the mixing test of grinding aids of hematite ore based on uniform design[J]. Multipurpose Utilization of Mineral Resources, 2019(2):30-36. doi: 10.3969/j.issn.1000-6532.2019.02.006
-



 下载:
下载: