Experimental Study on the Adsorption of Heavy Metal Ions in Wastewater by Modified Bentonite
-
摘要:
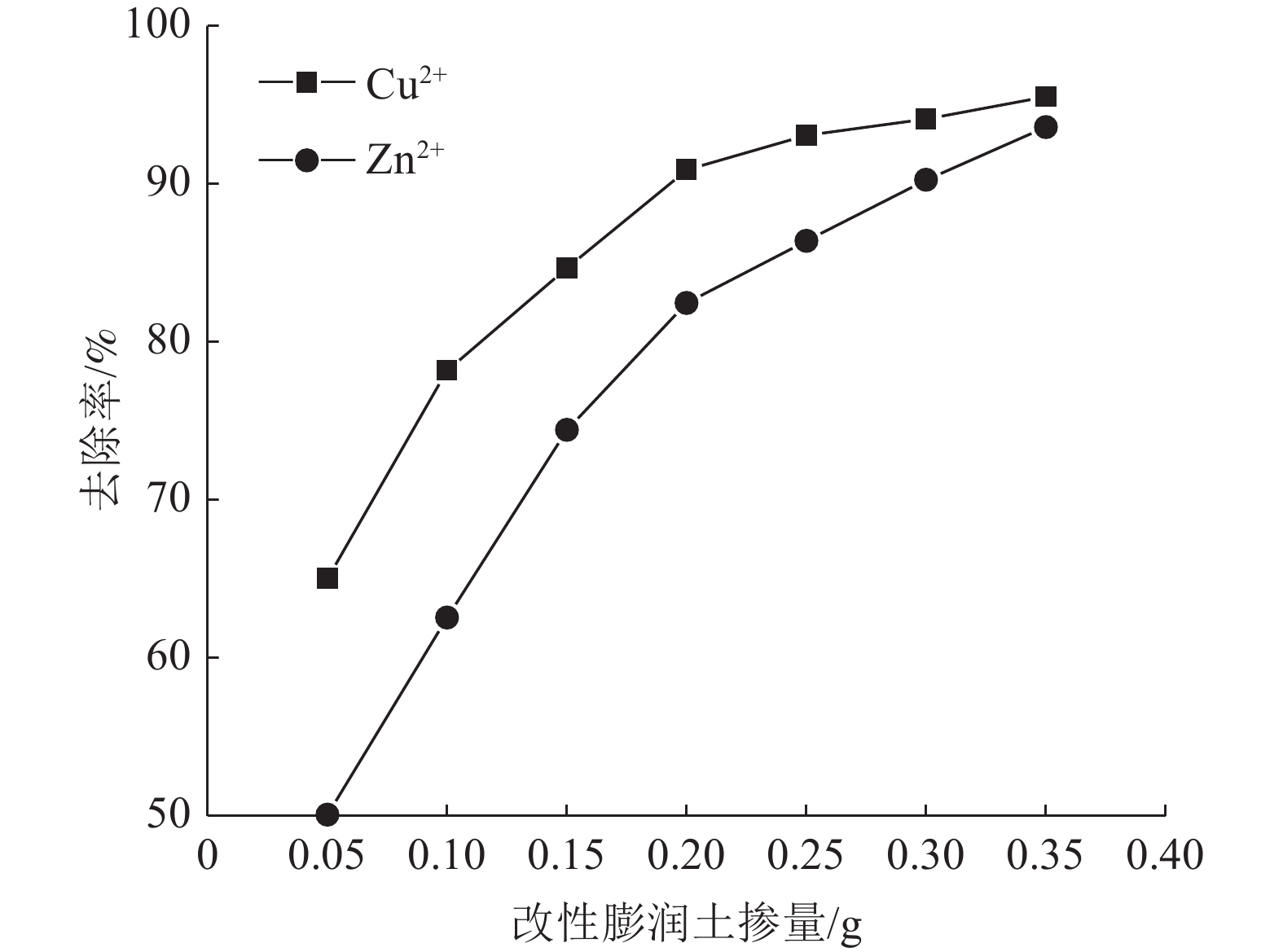
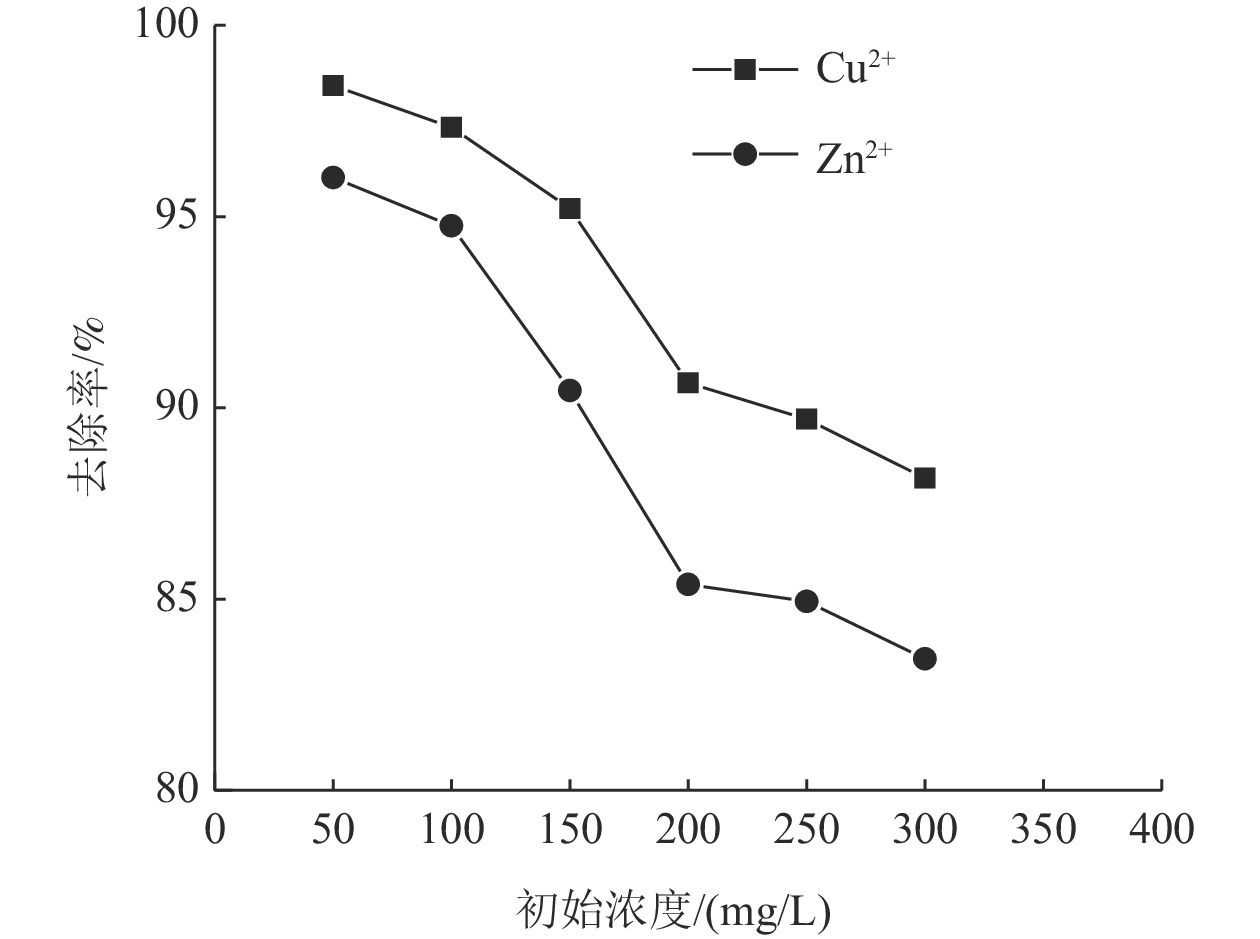
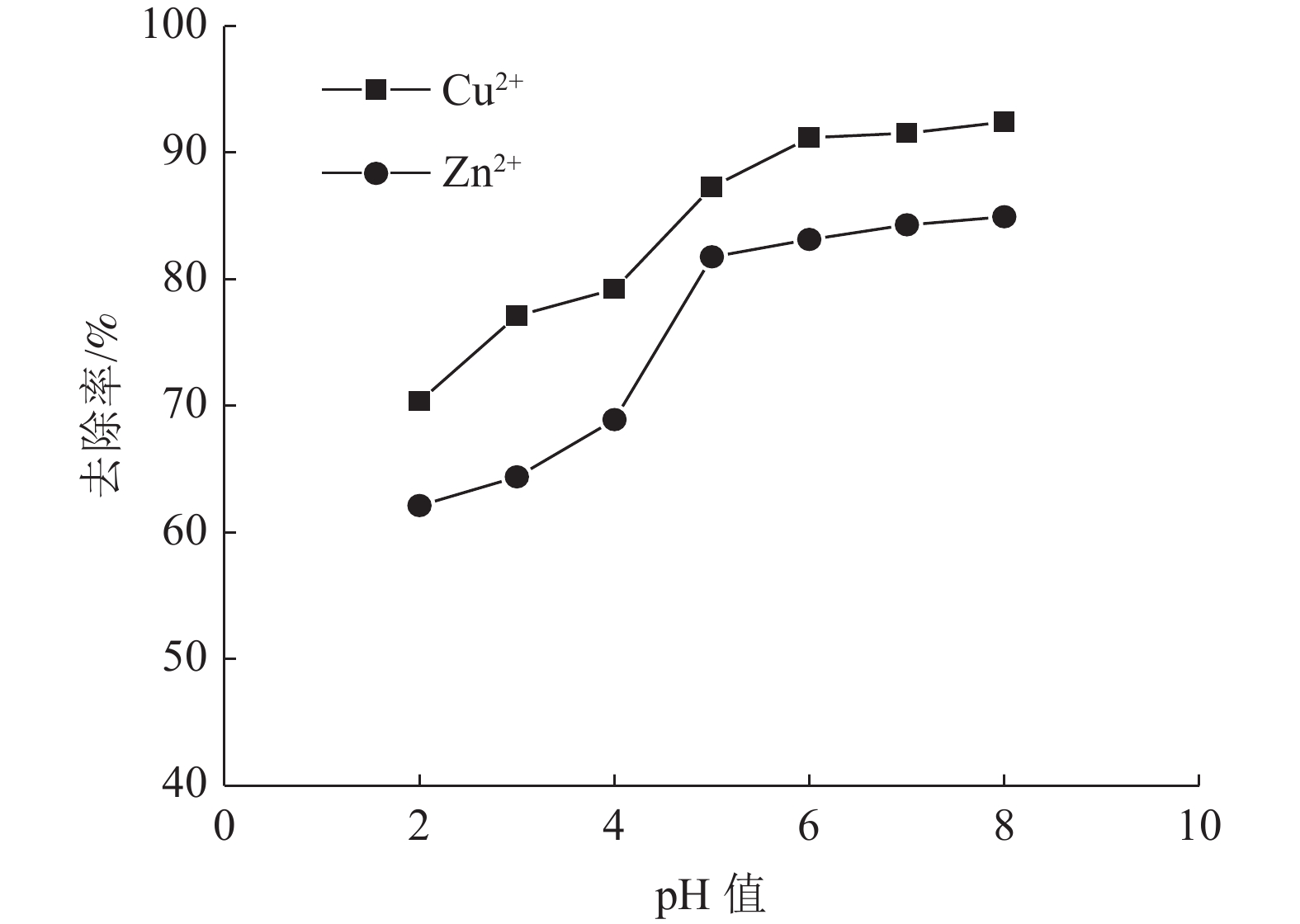
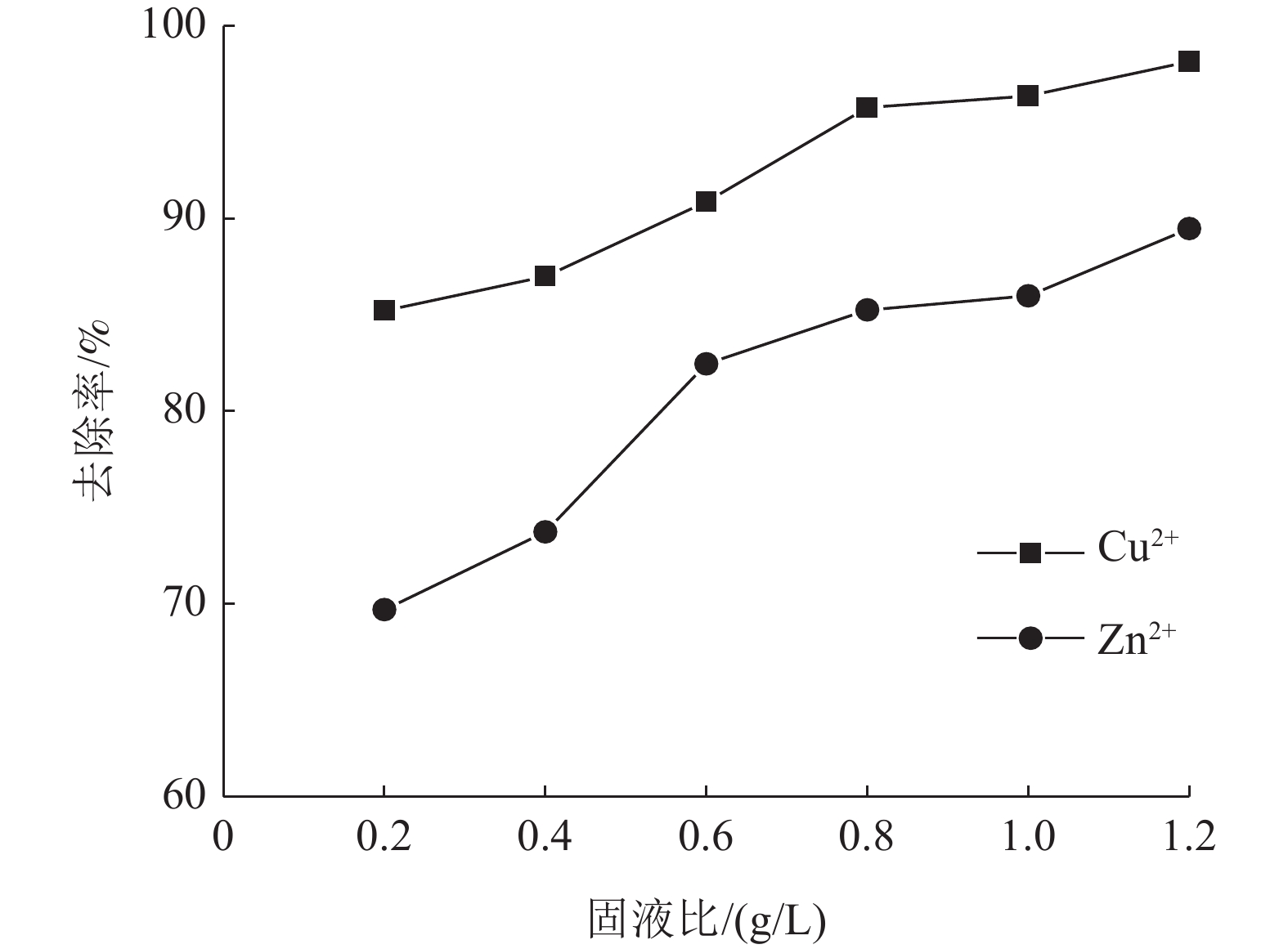
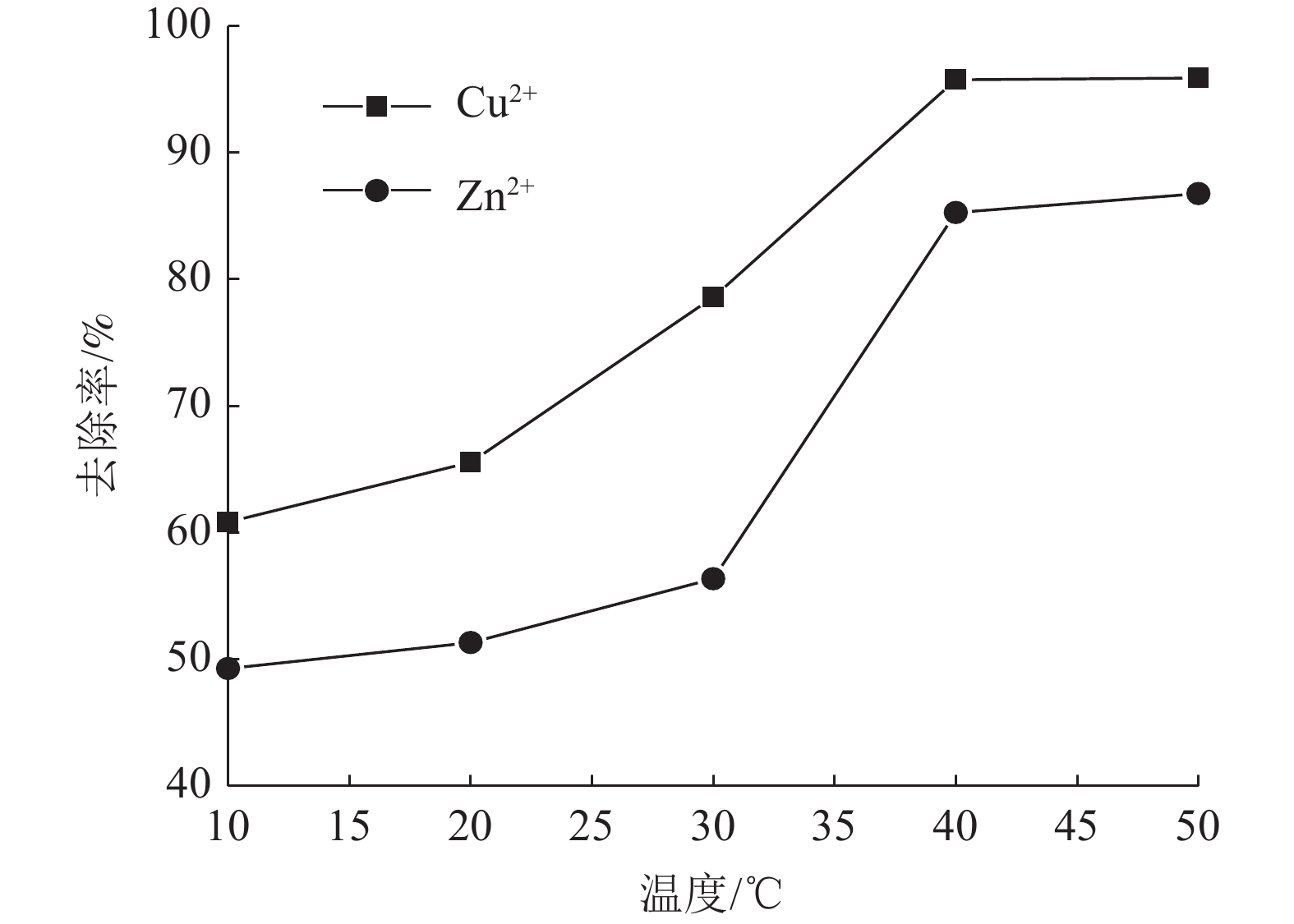
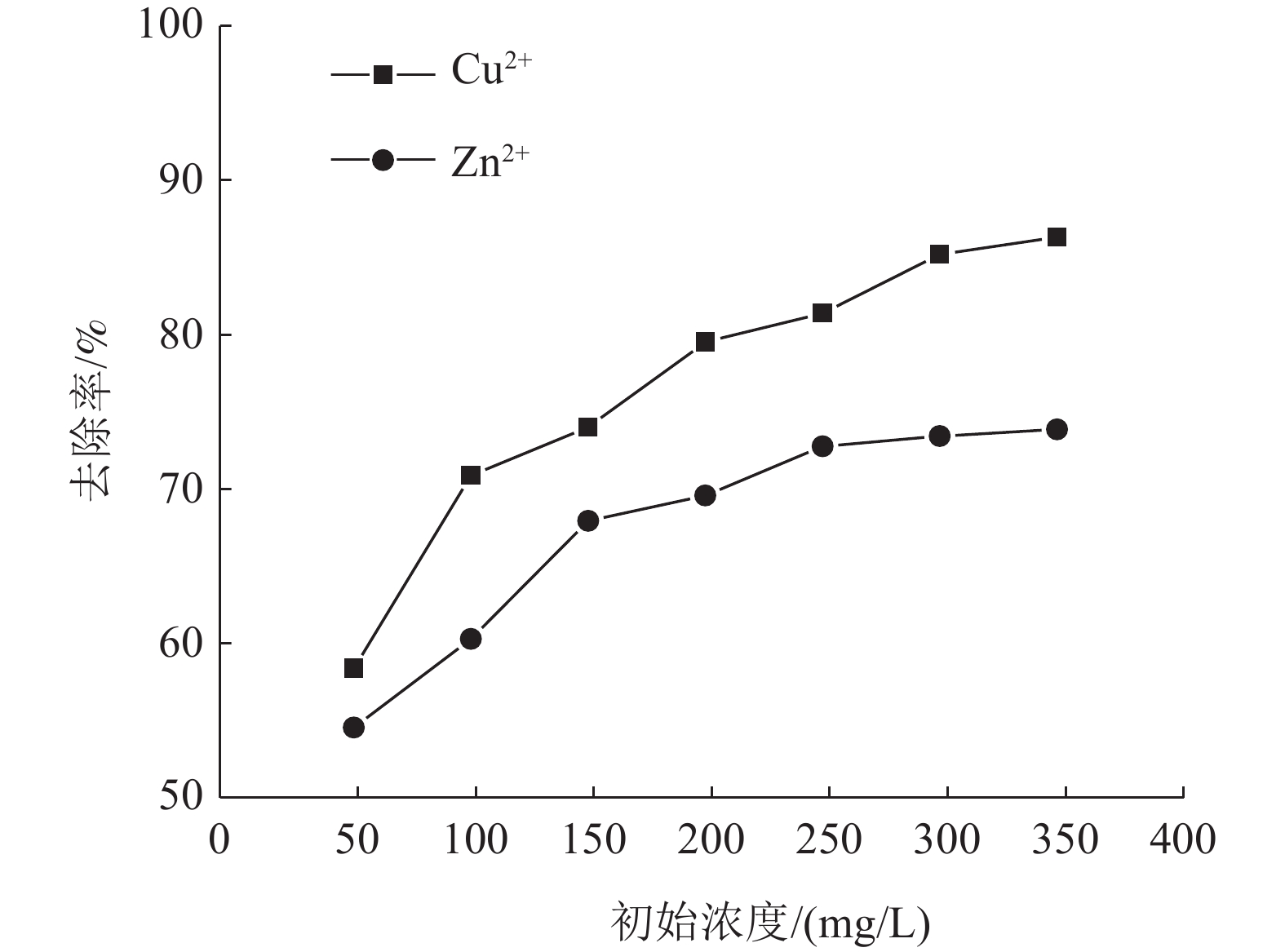
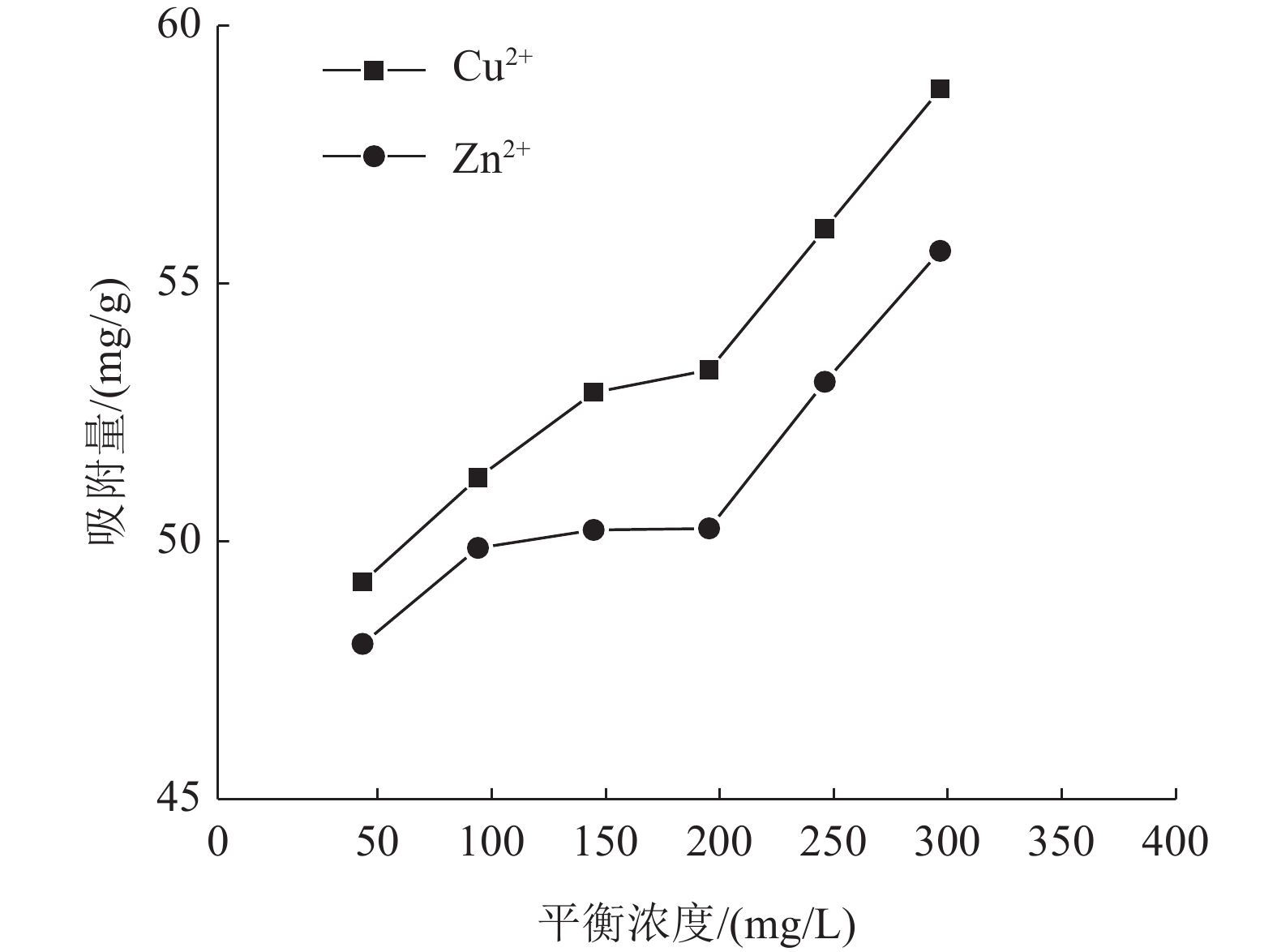
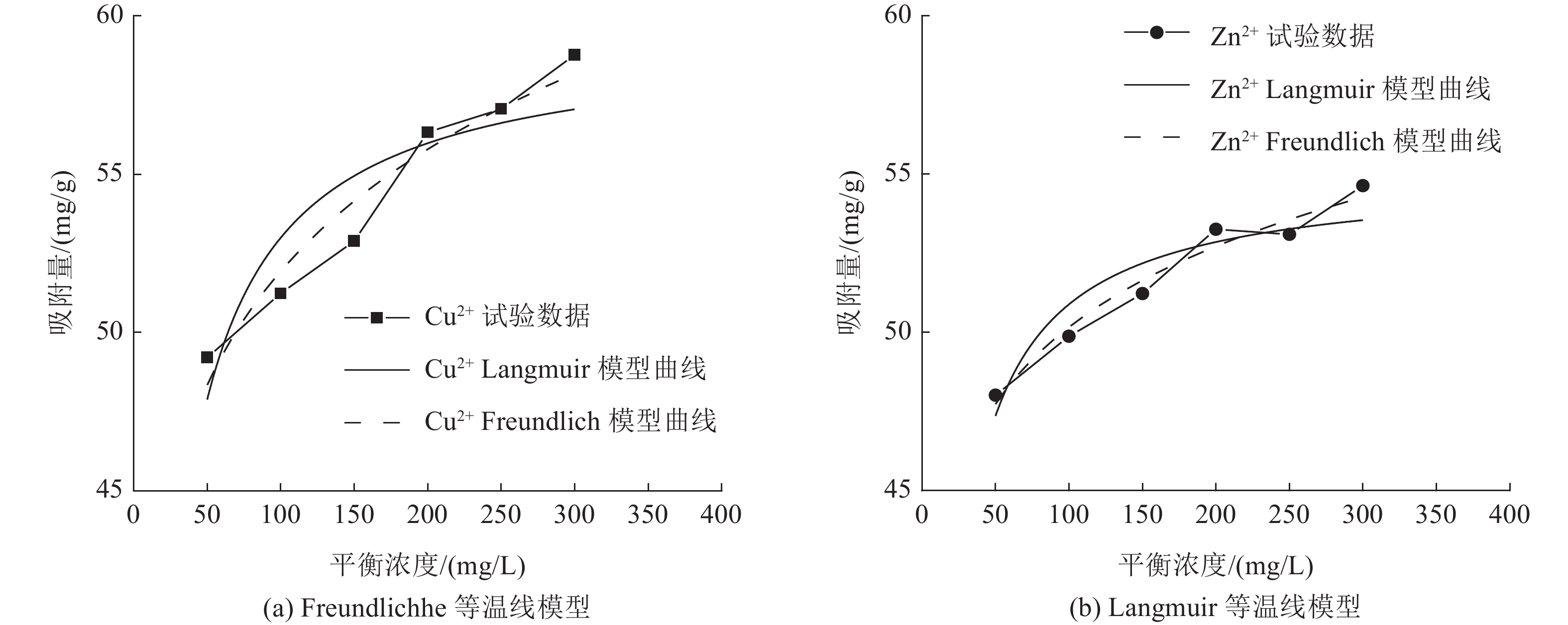
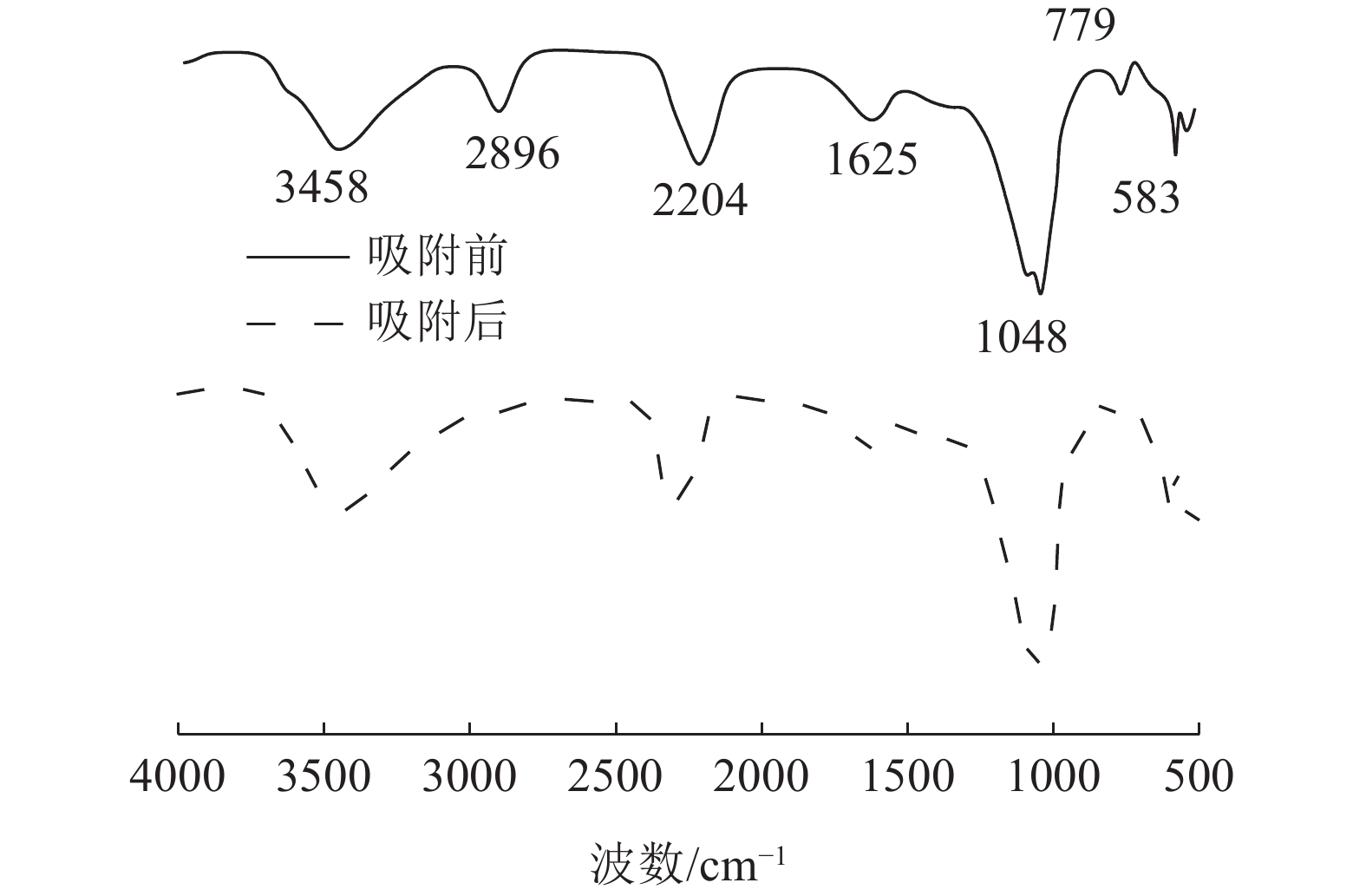
这是一篇陶瓷及复合材料领域的论文。为了研究改性膨润土对污水中重金属离子的吸附效果,分析pH值、初始浓度、时间、固液比、温度和粒径对膨润土吸附效果的影响,并采用微观手段研究在吸附重金属离子前后膨润土内部矿物成分的变化以及红外光谱图的变化规律。结果表明:当改性膨润土掺量为0.2 g、吸附时间为1.5 h、温度设置为40 ℃,pH值设定为6,初始浓度均设定为200 mg/L和固液比设置为0.8 g/L时,改性膨润土的吸附效果达到较佳。改性膨润土经过吸附实验后,可以检测到明显的CuSO4衍射峰,但是改性膨润土内部其他矿物成分不变,这说明改性膨润土可以有效地吸附污水中的铜离子。
Abstract:This is an article in the field of ceramics and composites. In order to study the adsorption effect of modified bentonite on heavy metal ions in sewage, the effects of pH value, initial concentration, time, solid-liquid ratio, temperature and particle size on the adsorption effect of bentonite were analyzed. And microscopic means were used to study the changes of the internal mineral composition of the bentonite before and after the adsorption of heavy metal ions and the change law of the infrared spectra. The results showed that when the modified bentonite content was 0.2 g, the adsorption time was 1.5 h, the temperature was set to 40 ℃, the pH value was set to 6, the initial concentration was set to 200 mg/L and the solid-to-liquid ratio was set to 0.8 g/L, the adsorption effect was the best. After the modified bentonite had undergone an adsorption test, an obvious CuSO4 diffraction peak could be detected. However, other mineral components inside the modified bentonite remained unchanged, which also showed that the modified bentonite could effectively adsorb copper ions in sewage.
-

-
[1] 郑长文, 管俊芳, 郑佳敏, 等. 矿业领域膨润土应用的研究进展[J]. 矿产综合利用, 2020(3):22-27.ZHENG C W, GUAN J F, ZHENG J M, et al. Progress in the application of bentonite in mining industry[J]. Multipurpose Utilization of Mineral Resources, 2020(3):22-27.
ZHENG C W, GUAN J F, ZHENG J M, et al. Progress in the application of bentonite in mining industry[J]. Multipurpose Utilization of Mineral Resources, 2020(3):22-27.
[2] 李遥, 时燕琳, 何建刚, 等. 铜对硒(Ⅳ)在高庙子膨润土中吸附行为的影响[J]. 环境化学, 2020, 39(2):334-342.LI Y, SHI Y L, HE J G, et al. Effect of copper on the adsorption behavior of selenium(IV) in Gaomiaozi bentonite[J]. Environmental Chemistry, 2020, 39(2):334-342.
LI Y, SHI Y L, HE J G, et al. Effect of copper on the adsorption behavior of selenium(IV) in Gaomiaozi bentonite[J]. Environmental Chemistry, 2020, 39(2):334-342.
[3] 汤鹏成, 傅开彬, 钟秋红, 等. 广西田东天然钙基膨润土对Cu(Ⅱ)的吸附性能研究[J]. 非金属矿, 2019, 42(6):77-81.TANG P C, FU K B, ZHONG Q H, et al. Study on the adsorption performance of natural calcium-based bentonite on Cu(II) in Tiandong, Guangxi[J]. Nonmetallic Mining, 2019, 42(6):77-81.
TANG P C, FU K B, ZHONG Q H, et al. Study on the adsorption performance of natural calcium-based bentonite on Cu(II) in Tiandong, Guangxi[J]. Nonmetallic Mining, 2019, 42(6):77-81.
[4] 郭争争, 管俊芳, 陈菲, 等. 重金属对膨润土膨胀性的影响[J]. 矿产综合利用, 2020(1):203-208.GUO Z Z, GUAN J F, CHEN F, et al. Effect of heavy metal ions on swelling property of bentonite[J]. Multipurpose Utilization of Mineral Resources, 2020(1):203-208.
GUO Z Z, GUAN J F, CHEN F, et al. Effect of heavy metal ions on swelling property of bentonite[J]. Multipurpose Utilization of Mineral Resources, 2020(1):203-208.
[5] 房斌, 张松, 江月, 等. 膨润土在河道治理中的应用[J]. 矿产综合利用, 2021(4):87-90.FANG B, ZHANG S, JIANG Y, et al. Study on the application of bentonite in river regulation[J]. Multipurpose Utilization of Mineral Resources, 2021(4):87-90.
FANG B, ZHANG S, JIANG Y, et al. Study on the application of bentonite in river regulation[J]. Multipurpose Utilization of Mineral Resources, 2021(4):87-90.
[6] 林娟, 姚佳雯, 魏笑, 等. 镧改性膨润土对磷吸附特性、机理与影响因素[J]. 环境科学与技术, 2021, 44(1):7-12.LIN J, YAO J W, WEI X, et al. Adsorption characteristics, mechanism and influencing factors of phosphorus adsorption by lanthanum-modified bentonite[J]. Environmental Science and Technology, 2021, 44(1):7-12.
LIN J, YAO J W, WEI X, et al. Adsorption characteristics, mechanism and influencing factors of phosphorus adsorption by lanthanum-modified bentonite[J]. Environmental Science and Technology, 2021, 44(1):7-12.
[7] 邹成龙, 聂发辉, 鲁秀国, 等. 羟基铝柱撑膨润土对水中Cr(Ⅵ)的吸附性能研究[J]. 化工新型材料, 2021, 49(1):184-189.ZOU C L, NIE F H, LU X G, et al. Adsorption performance of hydroxyaluminum columnar bentonite on Cr(VI) in water[J]. New Chemical Materials, 2021, 49(1):184-189.
ZOU C L, NIE F H, LU X G, et al. Adsorption performance of hydroxyaluminum columnar bentonite on Cr(VI) in water[J]. New Chemical Materials, 2021, 49(1):184-189.
[8] 孙志勇, 王智懿, 张娇, 等. 聚乙烯亚胺改性磁性膨润土对Pb~(2+)和Cu~(2+)的吸附性能[J]. 精细化工, 2021, 38(1):169-175+191.SUN Z Y, WANG Z Y, ZHANG J, et al. Adsorption properties of polyethyleneimine-modified magnetic bentonite on Pb~(2+) and Cu~(2+)[J]. Fine Chemical Industry, 2021, 38(1):169-175+191.
SUN Z Y, WANG Z Y, ZHANG J, et al. Adsorption properties of polyethyleneimine-modified magnetic bentonite on Pb~(2+) and Cu~(2+)[J]. Fine Chemical Industry, 2021, 38(1):169-175+191.
[9] 宋佳诺. 磁改性膨润土-钢渣复合材料对含Cu~(2+)、Zn~(2+)酸性矿山废水的处理[D]. 阜新: 辽宁工程技术大学, 2019.SONG J N. Treatment of acidic mine wastewater containing Cu~(2+) and Zn~(2+) by magnetically modified bentonite-steel slag composites[D]. Fuxin: Liaoning University of Engineering and Technology, 2019.
SONG J N. Treatment of acidic mine wastewater containing Cu~(2+) and Zn~(2+) by magnetically modified bentonite-steel slag composites[D]. Fuxin: Liaoning University of Engineering and Technology, 2019.
[10] 林志茂, 鲜东帆, 周万强, 等. U(Ⅵ)在缓冲回填材料高庙子膨润土胶体上的吸附[J]. 核化学与放射化学, 2021, 43(2):176-182.LIN Z M, XIAN D F, ZHOU W Q, et al. Adsorption of U(VI) on Gaomiaozi bentonite colloid, a buffer backfill material[J]. Nuclear Chemistry and Radiochemistry, 2021, 43(2):176-182.
LIN Z M, XIAN D F, ZHOU W Q, et al. Adsorption of U(VI) on Gaomiaozi bentonite colloid, a buffer backfill material[J]. Nuclear Chemistry and Radiochemistry, 2021, 43(2):176-182.
[11] 胡啸龙, 孟昭福, 王新欣, 等. 双子型复配增强两性膨润土吸附Cr(VI)的效应[J]. 中国环境科学, 2020, 40(5):2087-2094.HU X L, MENG Z F, WANG X X, et al. Enhancement of Cr(VI) adsorption effect of amphoteric bentonite by baryonic complexes[J]. China Environmental Science, 2020, 40(5):2087-2094.
HU X L, MENG Z F, WANG X X, et al. Enhancement of Cr(VI) adsorption effect of amphoteric bentonite by baryonic complexes[J]. China Environmental Science, 2020, 40(5):2087-2094.
-



 下载:
下载: