Characteristics and Distribution of Geothermal-type Lithium Resources in Southern Xizang
-
摘要:
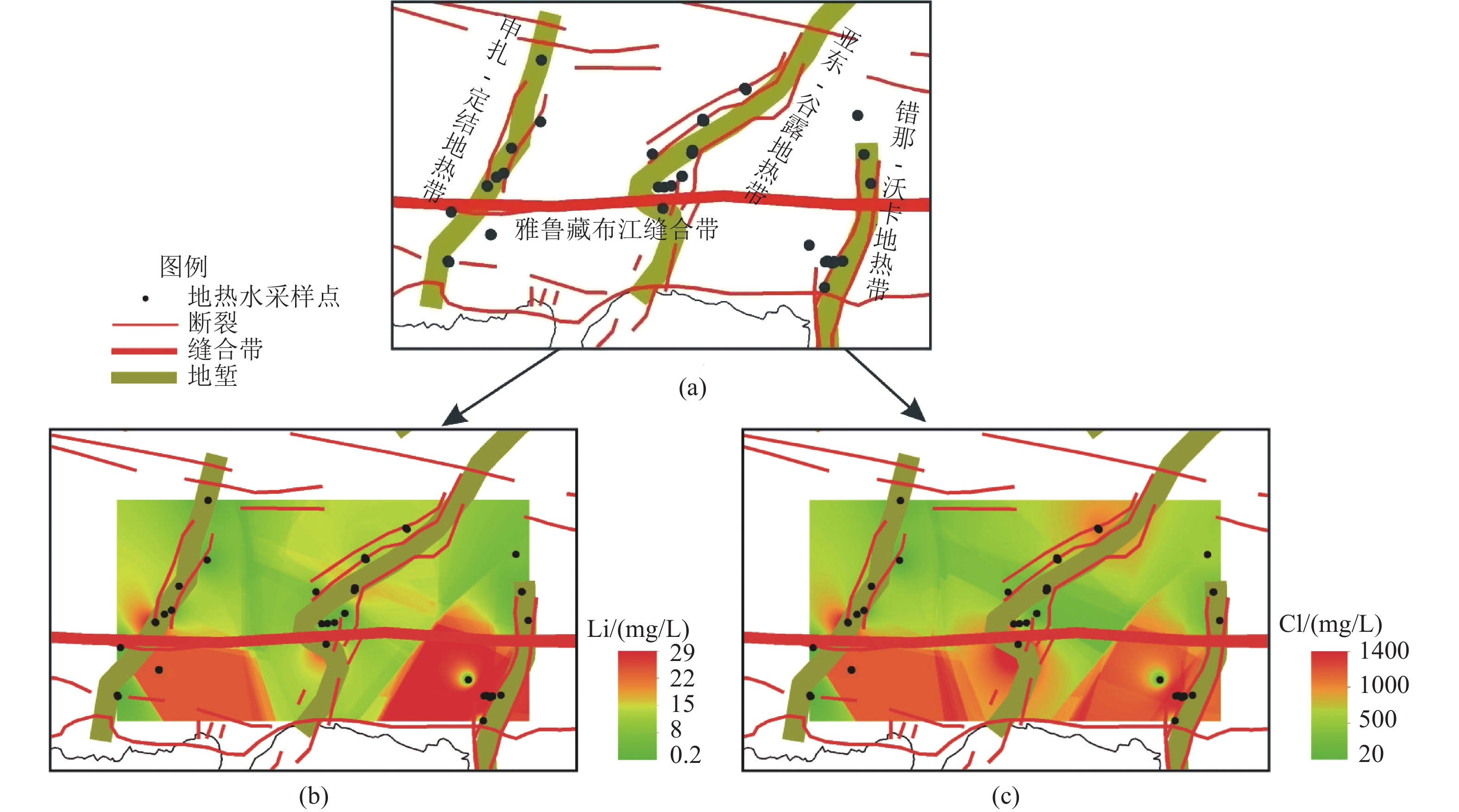
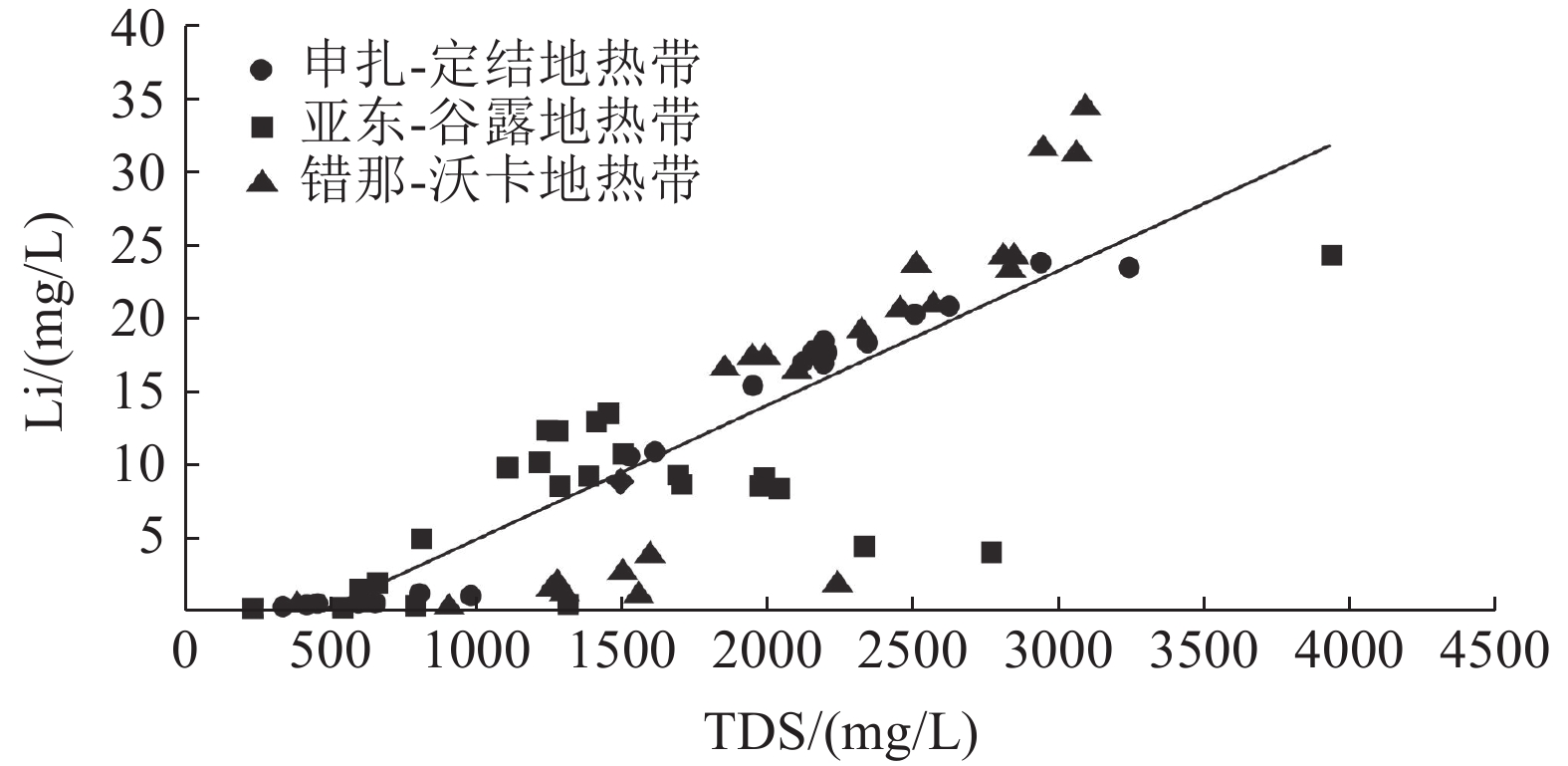
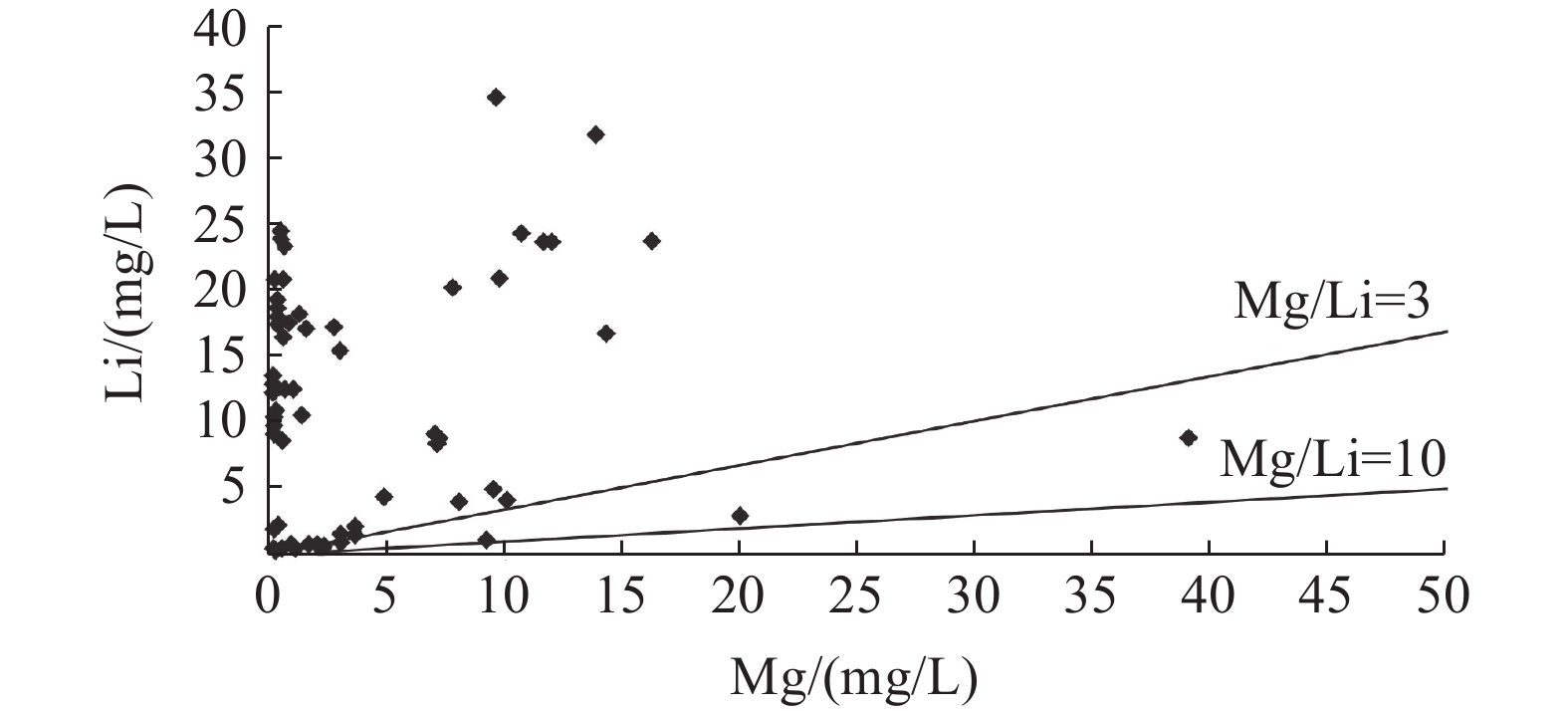
这是一篇矿业工程领域的论文。西藏南部是我国高温地热带的主要分布区之一,拥有丰富的地热资源。地热水化学分析测试结果显示,西藏南部高温地热带地热水中的锂含量最高可达34.51 mg/L,地热锂的相对丰度明显优于北美西部高原的克莱顿谷和南美安第斯高原的乌尤尼等世界典型的高原型盐湖卤水锂的相对丰度的盐湖卤水,镁锂比大部分小于3,有利于卤水提锂。西藏南部高温地热带地热水中锂含量较高的区域均分布在雅鲁藏布江缝合带及其以南地区,与富锂岩石的分布范围一致,同时雅鲁藏布江缝合带及其以南地区地热水的Cl-Na型地热水比北部的更多,南部地热水的TDS更高,循环路径更长。根据地热水的氢氧稳定同位素和周围富锂盐湖卤水中锂的物源推断了地热水锂的物源主要有两种,分别是地热水对富锂岩石的溶滤作用和岩浆分异过程中形成的富锂岩浆热液。
Abstract:This is an article in the field of mining engineering. The Southern Xizang is one of the main distribution areas of high temperature geothermal zones in China with rich geothermal resources. The chemical analysis and test results of the geothermal water show that the lithium content in the geothermal water of the high temperature geothermal zone in Southern Xizang can reach 34.51 mg/L, and the relative abundance of geothermal lithium is obviously better than that of the world's typical plateau Salt Lake brines, such as Clayton Valley in the Western Plateau of North America and Uyuni in the Andes Plateau of South America. The lithium magnesium ratio is mostly less than 3, which is conducive to lithium extraction from brine. The areas with high lithium content in the geothermal water of the high temperature geothermal zones in Southern Xizang are all distributed in the the Yarlung Zangbo River suture zone and the areas to the south, consistent with the distribution range of lithium rich rocks. At the same time, the Yarlung Zangbo River suture zone and its south area have more Cl-Na geothermal water than the north, and the south has higher TDS and longer circulation path. Based on the stable isotopic composition of hydrogen and oxygen in geothermal water and the provenance of lithium in the surrounding lithium-rich Salt Lake brine, it is inferred that the main sources of lithium in the geothermal water are leaching of lithium-rich rocks by geothermal water and lithium-rich magmatic hydrothermal solutions formed during magmatic differentiation.
-

-
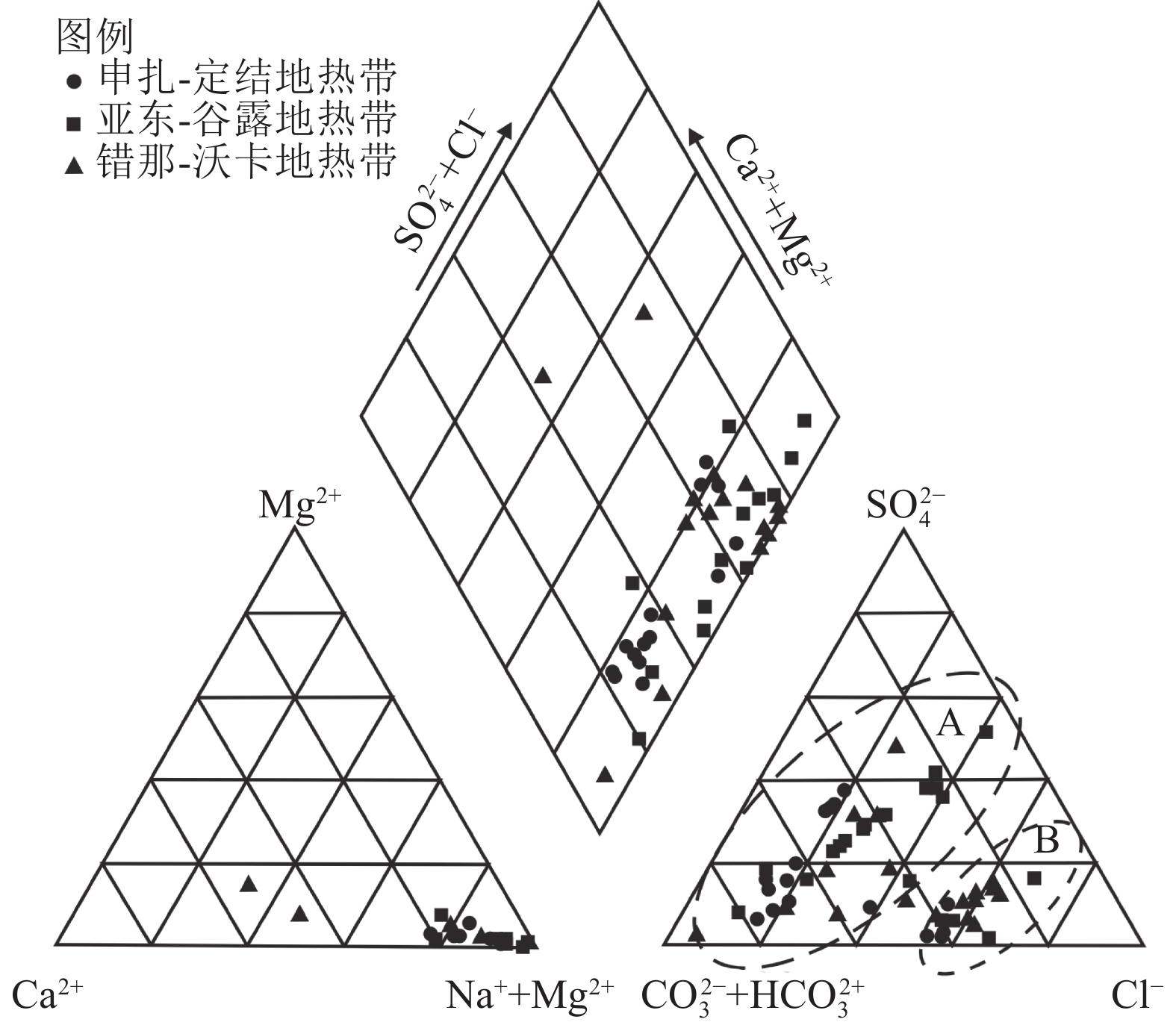
图 3 西藏南部地热带地热水piper[20]
Figure 3.
表 1 西藏南部代表性地热水化学组分分析结果
Table 1. Chemical composition analysis results of representative geothermal water in Southern Xizang
地热带 样品
编号pH值 温度/℃ 水化学类型 浓度/(mg/L) TDS Na+ K+ Mg2+ Ca2+ Cl- HCO3- SO42- CO32- Li 申扎-定结
地热带G101 9.17 86.0 Na-Cl·HCO3 2 211 561.50 72.44 1.23 3.88 595.43 416.24 30.83 143.58 17.65 G102 9.27 82.0 Na-SO4·HCO3 418 101.32 4.03 0.33 1.97 29.76 88.13 77.25 14.29 0.339 G103 8.21 84.0 Na-HCO3·Cl 1 612 422.61 48.68 0.11 3.11 276.07 635.68 83.56 0.00 10.78 G104 8.04 69.0 Na-HCO3 814 226.07 22.83 3.02 25.96 64.28 529.42 79.88 0.00 0.96 G105 7.18 77.0 Na-HCO3 988 291.37 23.01 9.25 36.67 91.88 782.13 48.39 0.00 1.12 G106 6.77 59.0 Na-Cl·HCO3 2 929 680.00 70.78 11.95 107.68 797.00 774.10 165.43 0.00 23.84 G107 6.86 28.5 Na-Cl·HCO3 2 614 584.88 68.08 9.78 120.16 660.25 755.53 144.92 0.00 20.74 G108 7.68 28.2 Na-Cl·HCO3 3 241 719.08 70.27 16.28 164.18 873.81 867.54 189.60 0.00 23.67 G109 7.25 44.5 Na-Cl·HCO3 2 503 576.22 59.82 7.80 104.52 682.28 682.39 139.39 0.00 20.1 G110 7.68 47.6 Na-HCO3·Cl 419 120.29 6.36 1.50 19.26 47.97 287.31 25.60 0.00 0.352 G111 7.86 62.0 Na-HCO3 653 181.17 9.55 2.27 25.01 56.77 345.22 88.05 0.00 0.593 G112 7.64 60.1 Na-HCO3 623 182.18 9.73 1.59 20.63 57.53 352.10 67.62 0.00 0.577 G113 8.29 40.3 Na-HCO3 601 184.33 9.82 0.85 14.88 56.67 365.56 35.31 8.88 0.621 G114 7.91 60.2 Na-HCO3·Cl 417 121.21 6.76 0.56 15.73 47.59 259.27 30.89 0.00 0.427 亚东-谷露
地热带G201 8.7 75.0 Na-SO4·Cl 608 142.93 7.73 0.21 10.57 88.57 72.39 123.80 10.73 1.45 G202 8.44 77.0 Na-SO4·Cl·HCO3 613 157.80 7.36 0.46 8.91 93.22 93.91 129.31 10.98 1.475 G203 7.34 49.0 Na-Cl·SO4·HCO3 2 767 731.57 34.84 10.13 164.51 573.88 597.05 696.37 0.00 4.015 G204 8.83 50.0 Na-SO4·Cl 805 215.91 4.77 0.09 13.52 141.15 27.71 235.13 6.80 0.524 G205 7.64 86.9 Na-Cl·HCO3 1 385 391.16 42.73 0.10 2.41 339.44 413.42 53.96 0.00 9.068 G206 9.5 82.3 Na-SO4·Cl·HCO3 1 295 329.76 21.08 0.41 2.76 145.47 242.16 202.91 86.50 8.511 G207 8.77 79.8 Na-Cl·HCO3 1 313 357.49 42.35 0.22 2.82 331.65 289.48 45.98 40.05 0.425 G208 9.11 - Na-HCO3·Cl·SO4 1 454 360.45 33.44 0.10 3.79 152.29 389.91 203.32 53.55 13.31 亚东-谷露
地热带G209 8.02 85.4 Na-HCO3·Cl 817 179.31 13.34 9.57 33.49 84.28 422.26 85.96 0.00 4.88 G210 7 26.5 Na-HCO3·Cl·SO4 1 517 398.75 36.73 0.22 9.76 151.65 606.10 202.67 0.00 10.71 G211 7.75 85.0 Na-HCO3·Cl·SO4 1 244 364.46 20.08 0.74 16.00 149.88 529.17 200.08 0.00 12.52 G212 9.32 79.8 Na-SO4·HCO3·Cl 1 264 326.17 23.50 0.57 3.07 149.40 258.27 208.88 65.87 12.28 G213 9.2 86.1 Na-HCO3·SO4·Cl 1 249 322.99 18.61 0.09 3.26 146.67 281.90 205.45 54.12 12.33 G214 8.5 85.8 Na-HCO3·Cl·SO4 1 115 291.53 15.51 0.09 2.23 143.12 262.30 191.49 20.73 9.744 G215 9.59 50.4 Na-SO4·Cl·HCO3·CO3 1 431 374.81 20.72 0.10 1.97 170.66 242.20 231.87 102.02 13.01 G216 9.2 46.3 Na-HCO3·Cl·SO4 1 214 316.32 16.55 0.22 6.78 147.00 270.95 196.92 60.61 10.38 G217 8.87 50.2 Na-HCO3 245 46.10 3.42 0.04 1.36 10.23 88.26 7.43 9.57 0.064 G218 9.02 51.8 Na-SO4·Cl 669 174.36 7.09 0.10 6.34 100.41 66.05 153.30 20.57 1.872 G219 6.92 54.7 Na-HCO3 2 335 801.63 37.96 4.88 53.50 161.53 1 592.19 344.02 0.00 4.352 G220 9.1 44.0 Na-Cl 552 173.05 1.77 0.09 7.11 191.03 37.81 60.30 15.18 0.3 错那-沃卡
地热带G301 6.95 80.0 Na-HCO3·Cl 1 602 459.57 46.31 8.04 49.42 276.56 867.86 88.19 0.00 3.928 G302 8.34 73.8 Na-Cl·HCO3 2 875 673.25 67.06 0.24 4.59 591.50 460.24 142.23 53.20 23.94 G303 8.83 74.9 Na-Cl·HCO3 2 828 648.05 73.19 0.49 4.22 574.94 373.27 131.38 114.52 24.4 G304 8.33 87.3 Na-Cl·HCO3 2 856 638.55 83.13 0.57 3.91 588.62 393.06 146.59 52.86 23.38 G305 7.61 43.1 Na-HCO3 917 339.52 16.04 2.06 14.11 35.41 948.61 18.13 0.00 0.562 G306 7.66 72.1 Na-Cl·HCO3 2 459 563.14 72.56 0.21 9.45 539.21 457.70 147.03 0.00 20.68 G307 7.83 80.02 Na-Cl·HCO3 2 329 535.50 68.60 0.20 4.36 533.89 318.82 148.90 21.53 19.16 G308 8.78 82.0 Na-Cl 1 954 471.81 56.55 0.81 5.16 479.27 189.80 151.02 68.41 17.51 G309 8.7 72.1 Na-Cl 1 994 480.01 65.79 0.82 5.61 491.40 211.43 154.07 60.81 17.49 G310 7.69 60.4 Na-Cl·HCO3 1 856 463.14 51.26 0.54 18.72 464.82 326.12 148.11 0.00 16.35 G311 6.78 56.3 Na-Cl·HCO3 2 513 581.56 67.19 11.64 81.07 625.84 780.68 130.44 0.00 23.71 G312 6.99 67.0 Na-Cl·HCO3 2 090 480.76 50.86 14.33 87.20 458.02 779.45 145.21 0.00 16.52 G313 6.5 30.0 Ca·Na-HCO3·Cl 1 496 159.23 27.23 39.15 237.27 207.74 821.91 203.71 0.00 8.815 G314 6.91 53.0 Na-Cl·HCO3 3 089 720.52 79.66 9.66 98.37 921.83 872.09 91.27 0.00 34.51 G315 7.06 64.2 Na-Cl·HCO3 2 958 671.50 91.29 13.92 122.86 844.08 823.35 119.99 0.00 31.67 G316 6.6 32.8 Na-Cl·HCO3 3 053 680.65 93.40 13.88 133.12 862.38 834.95 132.96 0.00 31.53 G317 6.87 63.0 Ca·Na-SO4·HCO3·Cl 1 512 217.69 27.38 20.05 195.78 189.40 382.83 503.10 0.00 2.855 G318 8.14 80.0 Na-HCO3·Cl 1 557 529.87 12.00 3.05 12.36 185.89 1 032.90 94.21 0.00 1.372 地点 盐湖/地热田 TDS/(g/L) Li/% Li/TDS (相对丰度) Mg/Li 北美西部高原 克莱顿谷 186 0.023 0.001 237 1.43 大盐湖 202 0.004 0.000 198 2.50 索尔顿海 293 0.026 6 0.000 908 0.16 南美安第斯高原 乌尤尼 231 0.05 0.002 165 8.40 阿塔卡玛 206 0.15 0.007 282 6.40 霍姆布雷托 254 0.062 0.002 441 1.40 中国青藏高原 班戈湖 68.5 0.010 4 0.104 0.64 扎仓茶卡 210 0.042 6 0.002 029 15.96 察尔汗盐湖 358 0.012 4 0.000 346 517.34 一里坪 327 0.026 2 0.000 801 92.30 西藏南部地热水 谢通门 2.929 0.002 384 0.008 139 0.50 措美-古堆 2.875 0.002 394 0.008 327 0.01 羊易 3.089 0.003 451 0.011 172 0.28 盐湖卤
水分类富锂、低镁锂比
碳酸盐型卤水中低镁锂
比盐湖卤水高镁锂比盐湖卤水 低锂、高镁锂
比盐湖卤水Mg/Li比 小于0.1 0.1~10 10~100 10~100 合适的提锂技术 盐梯度太阳池
提锂法(盐析法)分步沉淀法 煅烧法 萃取法 电渗析膜法 纳滤膜法 吸附法 代表
盐湖青藏高原扎
布耶盐湖智利
阿塔
卡玛盐湖青海
西台
吉乃尔盐湖青海
大柴
旦盐湖青海
东台吉
乃尔盐湖青海
西台
吉乃尔盐湖青海
察尔汗盐湖成本 2万元/t 2万元/t / 5万元/t 3万元/t 3万元/t 3万元/t -
[1] 王芳. 锂矿资源研究[D]. 北京: 中国地质大学(北京), 2001.WANG F. Research on lithium mineral resources[D]. Beijing: China University of Geosciences (Beijing), 2001.
WANG F. Research on lithium mineral resources[D]. Beijing: China University of Geosciences (Beijing), 2001.
[2] 李成秀, 程仁举, 刘星. 我国锂辉石选矿技术研究现状及展望[J]. 矿产综合利用, 2021(5):1-8.LI C X, CHENG R J, LIU X. Research status and prospects of spodumene ore beneficiation technology in China[J]. Multipurpose Utilization of Mineral Resources, 2021(5):1-8. doi: 10.3969/j.issn.1000-6532.2021.05.001
LI C X, CHENG R J, LIU X. Research status and prospects of spodumene ore beneficiation technology in China[J]. Multipurpose Utilization of Mineral Resources, 2021(5):1-8. doi: 10.3969/j.issn.1000-6532.2021.05.001
[3] 李晓波, 许浩, 王航, 等. 江西某钽铌尾矿中锂云母的浮选实验研究[J]. 矿产综合利用, 2023(5):36-40.LI X B, XU H, WANG H, et al. Flotation research on recovery of lithionite from Ta-Nb tailing in Jiangxi[J]. Multipurpose Utilization of Mineral Resources, 2023(5):36-40. doi: 10.3969/j.issn.1000-6532.2023.05.007
LI X B, XU H, WANG H, et al. Flotation research on recovery of lithionite from Ta-Nb tailing in Jiangxi[J]. Multipurpose Utilization of Mineral Resources, 2023(5):36-40. doi: 10.3969/j.issn.1000-6532.2023.05.007
[4] 吴西顺, 王登红, 杨添天, 等. 碳中和目标下的锂矿产业创新及颠覆性技术[J]. 矿产综合利用, 2022(2):1-8.WU X S, WANG D H, YANG T T, et al. Lithium mining industry innovation and disruptive technology under the goal of carbon neutrality[J]. Multipurpose Utilization of Mineral Resources, 2022(2):1-8. doi: 10.3969/j.issn.1000-6532.2022.02.001
WU X S, WANG D H, YANG T T, et al. Lithium mining industry innovation and disruptive technology under the goal of carbon neutrality[J]. Multipurpose Utilization of Mineral Resources, 2022(2):1-8. doi: 10.3969/j.issn.1000-6532.2022.02.001
[5] 何飞, 高利坤, 饶兵, 等. 从锂云母中提锂及综合利用的研究进展[J]. 矿产综合利用, 2022(5):82-89.HE F, GAO L K, RAO B, et al. Research progress on lithium extraction and comprehensive utilization from lepidolite[J]. Multipurpose Utilization of Mineral Resources, 2022(5):82-89.
HE F, GAO L K, RAO B, et al. Research progress on lithium extraction and comprehensive utilization from lepidolite[J]. Multipurpose Utilization of Mineral Resources, 2022(5):82-89.
[6] 徐璐, 杨耀辉, 颜世强, 等. 我国黏土型锂矿提锂研究现状及前景展望[J]. 矿产综合利用, 2023(4):12-18.XU L, YANG Y H, YAN S Q, et al. Lithium extraction from clay-type ore in China: status and prospects[J]. Multipurpose Utilization of Mineral Resources, 2023(4):12-18. doi: 10.3969/j.issn.1000-6532.2023.04.002
XU L, YANG Y H, YAN S Q, et al. Lithium extraction from clay-type ore in China: status and prospects[J]. Multipurpose Utilization of Mineral Resources, 2023(4):12-18. doi: 10.3969/j.issn.1000-6532.2023.04.002
[7] 刘成林, 余小灿, 袁学银, 等. 世界盐湖卤水型锂矿特征、分布规律与成矿动力模型[J]. 地质学报, 2021, 95(7):2009-2029.LIU C L, YU X C, YUAN X Y, et al. Characteristics, distribution regularity and formation model of brine-type Li deposits in salt lakes in the world[J]. Acta Geologica Sinica, 2021, 95(7):2009-2029. doi: 10.3969/j.issn.0001-5717.2021.07.001
LIU C L, YU X C, YUAN X Y, et al. Characteristics, distribution regularity and formation model of brine-type Li deposits in salt lakes in the world[J]. Acta Geologica Sinica, 2021, 95(7):2009-2029. doi: 10.3969/j.issn.0001-5717.2021.07.001
[8] 韩凤清. 青藏高原盐湖Li地球化学[J]. 盐湖研究, 2001, 9(1):55-61.HAN F Q. The geochemistry of lithium in salt lake on Qinghai-Tibetan Plateau[J]. Journal of salt lake research, 2001, 9(1):55-61.
HAN F Q. The geochemistry of lithium in salt lake on Qinghai-Tibetan Plateau[J]. Journal of salt lake research, 2001, 9(1):55-61.
[9] 王晨光, 郑绵平, 张雪飞, 等. 青藏高原南部地热型锂资源[J]. 科技导报, 2020, 38(15):24-36.WANG C G, ZHENG M P, ZHANG X F, et al. Geothermal-type lithium resources in Southern Tibetan Plateau[J]. Science & Technology Review, 2020, 38(15):24-36.
WANG C G, ZHENG M P, ZHANG X F, et al. Geothermal-type lithium resources in Southern Tibetan Plateau[J]. Science & Technology Review, 2020, 38(15):24-36.
[10] 王贵玲, 等. 中国地热志(西南卷三)[M]. 北京: 科学出版社, 2018: 145-150.WANG G L, et al. Geothermal records of China (southwest Volume III) [M]. Beijing: Science Press, 2018: 145-150.
WANG G L, et al. Geothermal records of China (southwest Volume III) [M]. Beijing: Science Press, 2018: 145-150.
[11] 王鹏, 陈晓宏, 沈立成, 等. 西藏地热异常区热储温度及其地质环境效应[J]. 中国地质, 2016, 43(4):1429-1438WANG P, CHEN X H, SHEN L C, et al. Reservoir temperature of geothermal anomaly area and its environmental effect in Tibet[J]. Geology in China, 2016, 43(4):1429-1438. doi: 10.12029/gc20160426
WANG P, CHEN X H, SHEN L C, et al. Reservoir temperature of geothermal anomaly area and its environmental effect in Tibet[J]. Geology in China, 2016, 43(4):1429-1438. doi: 10.12029/gc20160426
[12] 金胜. 青藏高原的壳幔电性结构特征及其动力学意义[D]. 北京: 中国地质大学(北京), 2009.JIN S. The characteristics of crust-mantle electrical structure and dynamics within Tibetan Plateau [D]. Beijing: China University of Geosciences (Beijing), 2009.
JIN S. The characteristics of crust-mantle electrical structure and dynamics within Tibetan Plateau [D]. Beijing: China University of Geosciences (Beijing), 2009.
[13] 李亚林, 王成善, 伊海生, 等. 青藏高原新生代地堑构造研究中几个问题的讨论[J]. 地质论评, 2005, 51(5):493-501.LI Y L, WANG C S, YI H S, et al. Current status and existing problems of lithium extraction technology from salt lake[J]. Geological Review, 2005, 51(5):493-501. doi: 10.3321/j.issn:0371-5736.2005.05.002
LI Y L, WANG C S, YI H S, et al. Current status and existing problems of lithium extraction technology from salt lake[J]. Geological Review, 2005, 51(5):493-501. doi: 10.3321/j.issn:0371-5736.2005.05.002
[14] 刘昭. 西藏尼木—那曲地热带典型高温地热系统形成机理研究[D]. 北京: 中国地质科学院, 2014.LIU Z. A Study on the formation mechanism of typical high temperature geothermal system in Nymu Naqudi Tropical Zone, Tibet [D]. Beijing: China University of Geosciences (Beijing), 2014.
LIU Z. A Study on the formation mechanism of typical high temperature geothermal system in Nymu Naqudi Tropical Zone, Tibet [D]. Beijing: China University of Geosciences (Beijing), 2014.
[15] 周训, 胡伏生, 何江涛, 等 . 地下水科学概论[M]. 北京: 地质出版社, 2014: 96-114.ZHOU X, HU F S, HE J T, et al. General outline of groundwater science[M]. Beijing: Geological Publishing House, 2014: 96-114.
ZHOU X, HU F S, HE J T, et al. General outline of groundwater science[M]. Beijing: Geological Publishing House, 2014: 96-114.
[16] 郑绵平. 青藏高原盐湖资源研究的新进展[J]. 地球学报, 2001, 22(2):97-102.ZHENG M P. Study advances in Saline Lake resources on the Qinghai Tibet Pleteau[J]. Acta Geoscientica Scinica, 2001, 22(2):97-102. doi: 10.3321/j.issn:1006-3021.2001.02.001
ZHENG M P. Study advances in Saline Lake resources on the Qinghai Tibet Pleteau[J]. Acta Geoscientica Scinica, 2001, 22(2):97-102. doi: 10.3321/j.issn:1006-3021.2001.02.001
[17] 李增荣, 刘国旺, 唐发满. 青海盐湖锂资源及提锂技术概述[J]. 资源信息与工程, 2017, 32(5):94-97.LI Z R, LIU G W, TANG F M. Overview of lithium resources and lithium extraction technology in Qinghai Salt Lake[J]. Resource information and engineering, 2017, 32(5):94-97. doi: 10.3969/j.issn.2095-5391.2017.05.045
LI Z R, LIU G W, TANG F M. Overview of lithium resources and lithium extraction technology in Qinghai Salt Lake[J]. Resource information and engineering, 2017, 32(5):94-97. doi: 10.3969/j.issn.2095-5391.2017.05.045
[18] 高峰, 郑绵平, 乜贞, 等. 盐湖卤水锂资源及其开发进展[J]. 地球学报, 2011, 32(4):483-492.GAO F, ZHENG M P, MIE Z, et al. Brine lithium resource in the salt lake and advances in its exploitation[J]. Acta Geoscientica Sinica, 2011, 32(4):483-492. doi: 10.3975/cagsb.2011.04.13
GAO F, ZHENG M P, MIE Z, et al. Brine lithium resource in the salt lake and advances in its exploitation[J]. Acta Geoscientica Sinica, 2011, 32(4):483-492. doi: 10.3975/cagsb.2011.04.13
[19] 李正山. 青海锂矿资源可持续开发路径研究-以柴达木-里坪项目为例[D]. 北京: 中国地质大学(北京), 2017.LI Z S. Study of Qinghai lithium mineral resources sustainable development path-in Qaidam a ping project as an example[D]. Beijing: China University of Geosciences (Beijing), 2017.
LI Z S. Study of Qinghai lithium mineral resources sustainable development path-in Qaidam a ping project as an example[D]. Beijing: China University of Geosciences (Beijing), 2017.
[20] Piper A M. A graphic procedure in the geochemical interpretation of wateranalyses. Eos Trans. Transactions American Geophysical Union, 1944, 25 (6), 914–928. http://dx. doi.org/10.1029/TR025i006p00914.
[21] 王学求, 刘汉粮, 王玮, 等. 中国锂矿地球化学背景与空间分布: 远景区预测[J]. 地球学报, 2020, 41(6):797-806.WANG X Q, LIU H L, WANG W, et al. Geochemical abundance and spatial distribution of lithium in China: implications for potential prospects[J]. Acta Geoscientica Scinica, 2020, 41(6):797-806. doi: 10.3975/cagsb.2020.081201
WANG X Q, LIU H L, WANG W, et al. Geochemical abundance and spatial distribution of lithium in China: implications for potential prospects[J]. Acta Geoscientica Scinica, 2020, 41(6):797-806. doi: 10.3975/cagsb.2020.081201
[22] 秦克章, 赵俊兴, 何畅通, 等. 喜马拉雅琼嘉岗超大型伟晶岩型锂矿的发现及意义[J]. 岩石学报, 2021, 37(11):3277-3286.QIN K Z, ZHAO J X, HE C T, et al. Discovery of the Qongjiagang giant lithium pegmatite deposit in Himalaya, Tibet, China[J]. Acta Petrologica Sinica, 2021, 37(11):3277-3286. doi: 10.18654/1000-0569/2021.11.02
QIN K Z, ZHAO J X, HE C T, et al. Discovery of the Qongjiagang giant lithium pegmatite deposit in Himalaya, Tibet, China[J]. Acta Petrologica Sinica, 2021, 37(11):3277-3286. doi: 10.18654/1000-0569/2021.11.02
[23] 赵俊兴, 何畅通, 秦克章, 等. 喜马拉雅琼嘉岗超大型伟晶岩锂矿的形成时代、源区特征及分异特征[J]. 岩石学报, 2021, 37(11):3325-3347.ZHAO J X, HE C T, QIN K Z, et al. Geochronology, source features and the characteristics of fractional crystallization in pegmatite at the Qiongjiagang giant pegmatite-type lithium deposit, Himalaya, Tibet[J]. Acta Geoscientica Scinica, 2021, 37(11):3325-3347. doi: 10.18654/1000-0569/2021.11.06
ZHAO J X, HE C T, QIN K Z, et al. Geochronology, source features and the characteristics of fractional crystallization in pegmatite at the Qiongjiagang giant pegmatite-type lithium deposit, Himalaya, Tibet[J]. Acta Geoscientica Scinica, 2021, 37(11):3325-3347. doi: 10.18654/1000-0569/2021.11.06
[24] 中国青藏高原综合科学考察队著. 西藏盐湖[M]. 北京: 科学出版社, 1988: 30.Research team of tibetan plateau comprehensive science. 1988. Tibet Salt Lake[M]. Beijing: Science Press, 1988: 30.
Research team of tibetan plateau comprehensive science. 1988. Tibet Salt Lake[M]. Beijing: Science Press, 1988: 30.
[25] 赵振华. 微量元素地球化学原理[M]. 北京: 科学出版社, 1997: 55.ZHAO Z H. 1997. Principles of trace element geochemistry[M]. Beijing: Science Press, 1997: 55.
ZHAO Z H. 1997. Principles of trace element geochemistry[M]. Beijing: Science Press, 1997: 55.
[26] WANG C G, ZHENG M P, ZHANG X F, et al. O, H, and Sr isotope evidence for origin and mixing processes of the Gudui geothermal system, Himalayas, China[J]. Geoscience Frontiers, 2019, 11:1175-1187.
-



 下载:
下载: