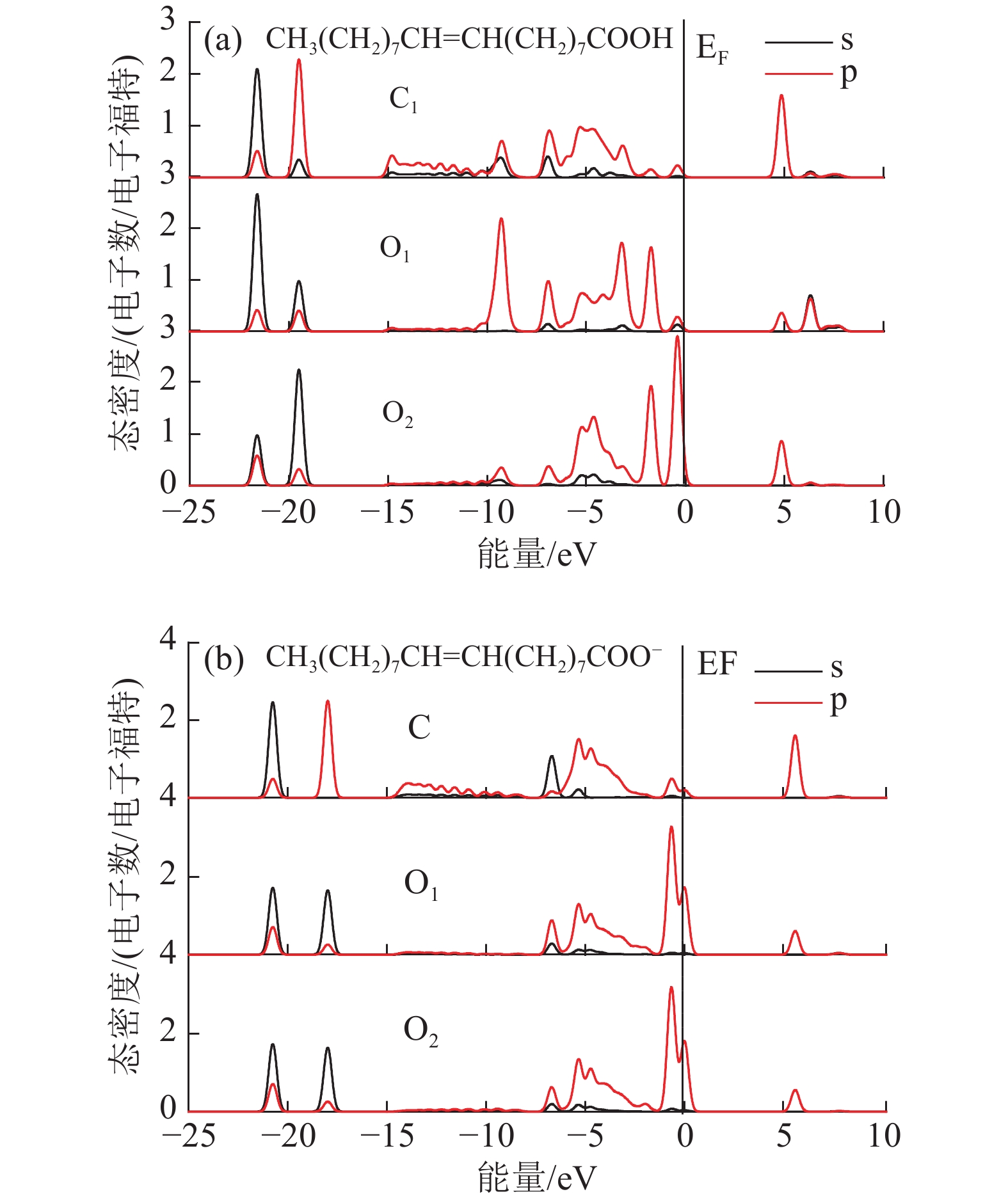
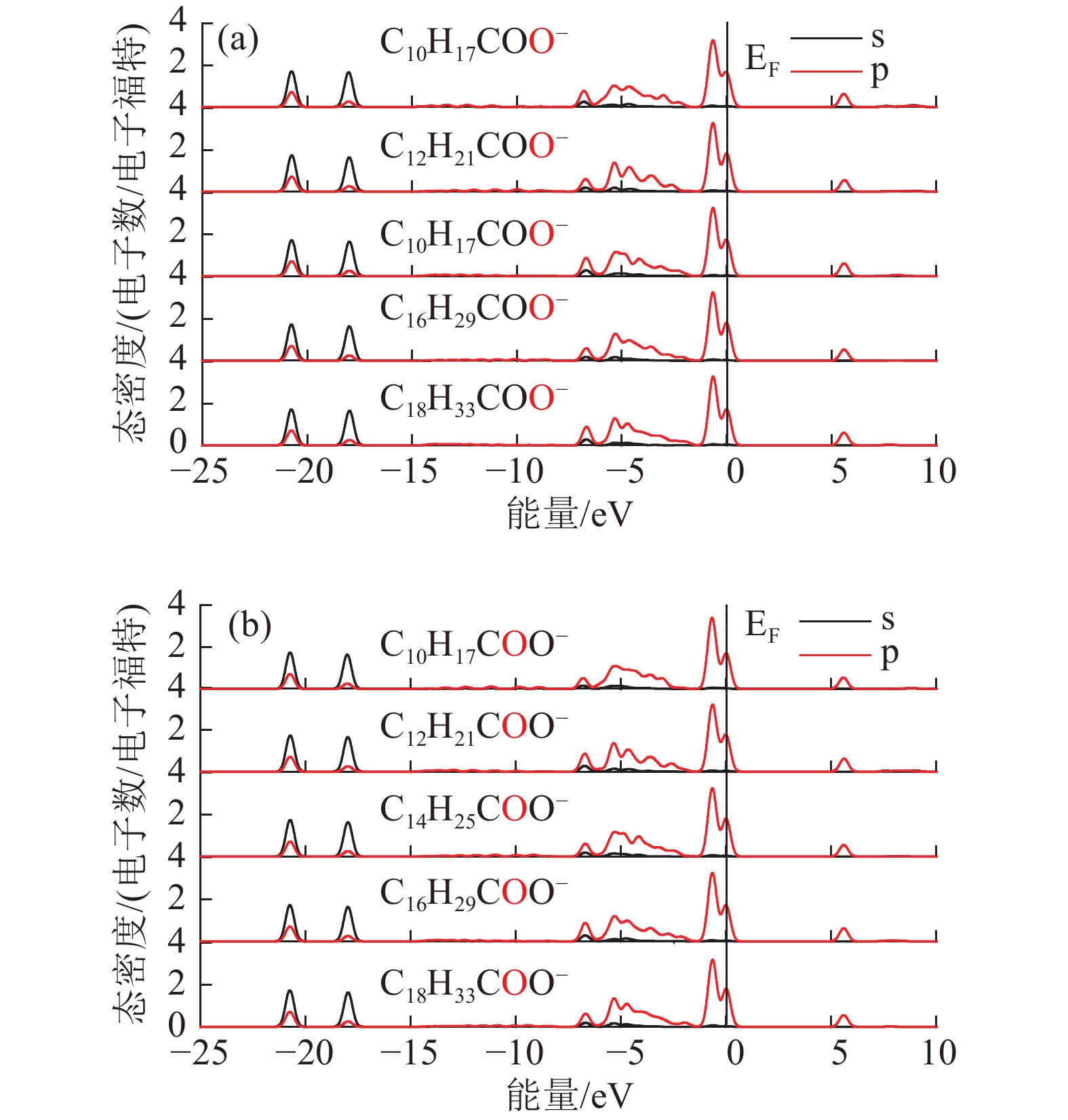
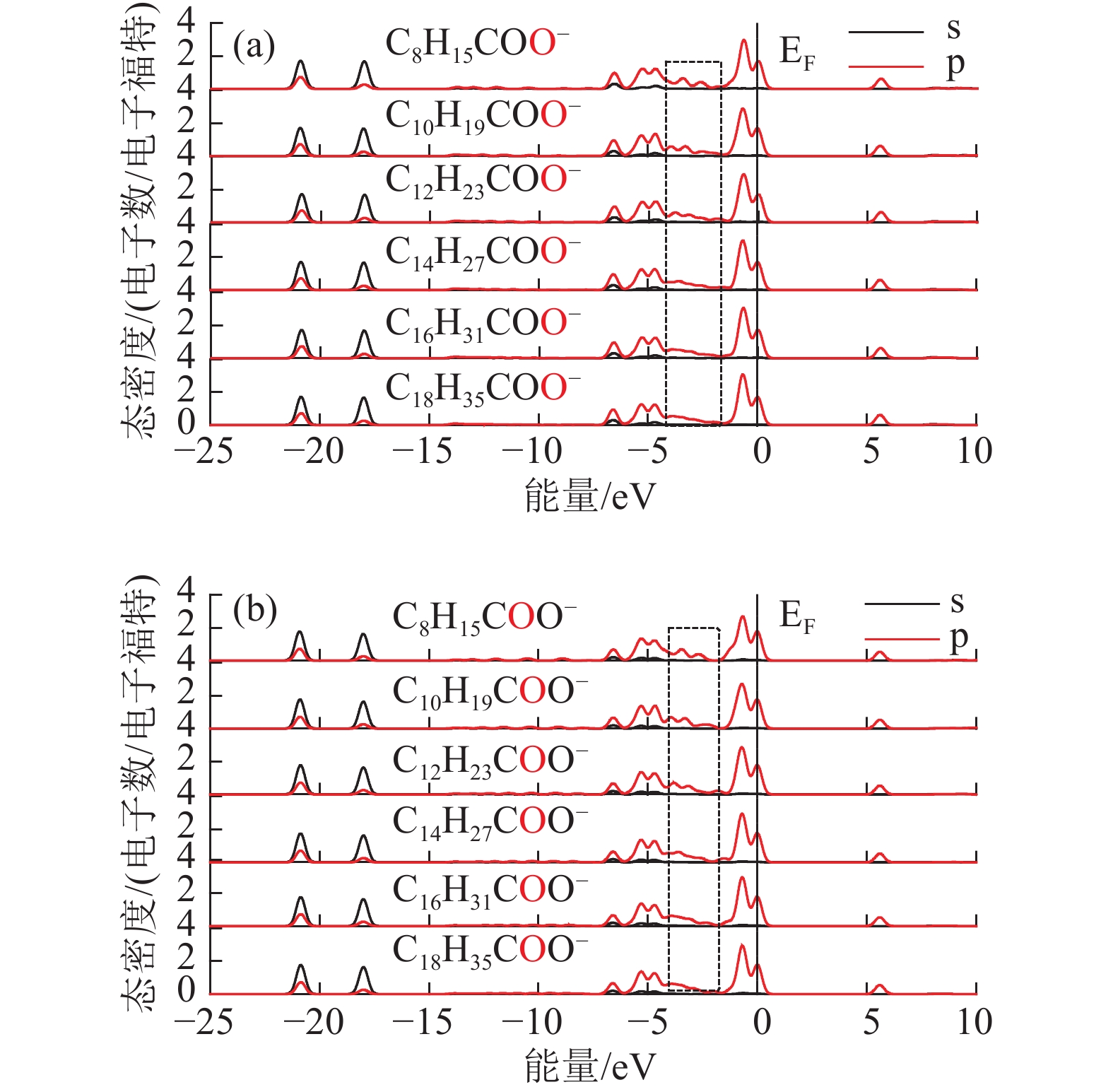
Density Functional Theory of Molecular Structure and Properties of Fatty Acid Collectors
-
摘要:
这是一篇矿物加工工程领域的论文。脂肪酸类捕收剂广泛应用于浮选氧化矿,其分子结构是影响浮选性能的重要因素,为了从微观角度揭示脂肪酸捕收剂结构变化对其反应活性的影响,采用密度泛函理论研究了脂肪酸电子结构与性能的关系。研究结果表明:油酸分子和油酸根离子中氧原子有很强的反应活性,氧原子是油酸与氧化矿物表面作用的键合原子;油酸根离子的两个氧原子具有相近的化学活性,且在费米能级处的态密度大于油酸分子,使得油酸根离子的活性明显强于油酸分子;碳链长度和烃基不饱和度几乎不影响脂肪酸中氧原子的态密度,其对脂肪酸性能的影响不是通过羧基作用的;脂肪酸与常见金属阳离子的相互作用能与其溶度积常数降低的规律一致,两者存在较好的对应关系;研究对了解脂肪酸捕收剂结构与性能的关系及开发新型脂肪酸类捕收剂有一定的理论意义和参考价值。
Abstract:This is an article in the field of mineral processing engineering. Fatty acid collectors are widely used in the flotation of oxidized ores, and the molecular structure is an important factor affecting the flotation performance. To reveal the effect of the structural change of fatty acid collector on its reaction activity from the microscopic point of view, the relationship between electronic structure and properties of fatty acid collectors was studied by the density functional theory. The research results show that the oxygen atom in oleic acid molecule and oleate ion has strong reaction activity, which is the bonding atom of oleic acid interaction with oxidized minerals. The two oxygen atoms of oleate ion have similar chemical activity, and the density of states at Fermi level is higher than that of oleic acid molecule, which makes the activity of oleate ion much stronger than that of oleic acid molecule. The length of the carbon chain and the unsaturation of the alkyl have almost no effect on the density of states of the oxygen atoms in fatty acids, their effect on the properties of fatty acids is not through the action of carboxyl groups. The interaction energy between fatty acids and common metal cations is consistent with the decrease of their solubility product constants, and there is a good corresponding relationship between them. The research has certain theoretical significance and reference value for understanding the relationship between the structure and performance of fatty acid collectors and developing new fatty acid collectors.
-

-
表 1 油酸在不同泛函和基组下几何结构优化后的性质参数
Table 1. Properties of oleic acid in different functionals and basis sets under the geometric optimization
基组泛函 键长/Å 键角/° 能量/Ha C1-C2 C1-O1 C1-O2 O1-C1-O2 DNP 3.5 LDA PWC 1.485 1.259 1.256 115.924 -848.80 DNP 3.5 LDA VWN 1.485 1.259 1.256 115.924 -848.84 DNP 3.5 GGA PW91 1.504 1.268 1.267 116.049 -856.15 DNP 3.5 GGA BP 1.508 1.270 1.270 116.071 -856.39 DNP 3.5 GGA BPE 1.506 1.269 1.269 115.976 -855.37 DNP 3.5 GGA BLYP 1.516 1.274 1.274 116.223 -856.09 DNP 3.5 GGA BOP 1.520 1.275 1.275 116.148 -856.06 DNP+ 3.5 LDA PWC 1.484 1.259 1.255 115.841 -848.85 DNP+ 3.5 LDA VWN 1.484 1.259 1.255 115.851 -848.89 DNP+ 3.5 GGA PW91 1.505 1.268 1.267 115.914 -856.20 DNP+ 3.5 GGA BP 1.508 1.270 1.269 115.773 -856.34 DNP+ 3.5 GGA PBE 1.506 1.270 1.269 115.811 -855.42 DNP+ 3.5 GGA BLYP 1.515 1.274 1.273 116.191 -856.14 DNP+ 3.5 GGA BOP 1.520 1.275 1.275 116.047 -856.10 实验数据 1.50 1.27 1.27 119 表 2 常见脂肪酸捕收剂及其双键数量
Table 2. Common fatty acid collectors and the number of double bonds
脂肪酸 分子式 双键数 硬脂酸 C17H35COOH 0 棕榈油 C15H31COOH 0 肉豆蔻酸 C13H27COOH 0 月桂酸 C11H23COOH 0 油酸 CH3(CH2)7CH=CH(CH2)7COOH 1 亚油酸 CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH 2 亚麻酸 CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH 3 桐酸 CH3(CH2)3CH=CHCH=CHCH=
CH(CH2)7COOH3 表 3 饱和脂肪酸与Ca2+作用能与碳链长度的关系
Table 3. Relationship between interaction energy of saturated fatty acids with Ca2+ and carbon chain lengths
饱和脂肪酸 能量/Ha 与Ca2+作用能/
(kJ/mol)溶度积
Ksp(23 ℃)[25]正辛酸 -464.41 -621.82 2.7×10-7 癸酸 -543.05 -626.37 3.8×10-10 月桂酸 -621.69 -630.36 8.0×10-13 肉豆蔻酸 -699.11 -644.91 1.0×10-15 棕榈酸 -778.97 -649.25 1.6×10-16 硬脂酸 -857.61 -653.94 1.4×10-18 表 4 油酸与常见金属阳离子的相互作用能与溶度积的关系
Table 4. Relationship between interaction energy and solubility product of oleic acid with common metal cations
金属阳离子 油酸/
(kJ/mol)能量/
(kJ/mol)相互作用能/
(kJ/mol)溶度积/
pKa [24]Mg2+ -856.388 -200.042 -419.751 13.8 Ba2+ -856.388 -7886.803 -496.144 14.9 Ca2+ -856.388 -677.519 -634.191 15.4 Zn2+ -856.388 -1779.366 -893.495 18.4 Cu2+ -856.388 -1640.487 -959.389 19.4 表 5 不同烃基不饱和度脂肪酸的物理性质[28]
Table 5. Physical property of different unsaturated fatty acids
脂肪酸 熔点/℃ 临界胶束浓度/(g/L) 烃基断面积/Å2 硬脂酸 65 0.00045 24.4 油酸 16.3 0.0012 56.6 亚油酸 -6.5 0.15 59.9 亚麻酸 -12.5 0.20 68.2 -
[1] 李靖, 张覃, 李龙江, 等. 采用浮选柱从中低品位磷矿石中富磷降镁初步试验研究[J]. 矿业研究与开发, 2017, 37(7):79-83.LI J, ZHANG Q, LI L J, et al. Preliminary experimental study on phosphorus enrichment and magnesium reduction from medium and low grade phosphate ores using flotation column[J]. Mining Research and Development, 2017, 37(7):79-83.
LI J, ZHANG Q, LI L J, et al. Preliminary experimental study on phosphorus enrichment and magnesium reduction from medium and low grade phosphate ores using flotation column[J]. Mining Research and Development, 2017, 37(7):79-83.
[2] 谢俊, 张覃. 氟磷灰石 Ca 位点成键特性的密度泛函理论研究[J]. 贵州科学, 2021, 39(1):89-96.XIE J, ZHANG Q. Density-functional theory study of bonding properties at Ca sites of fluorapatite[J]. Guizhou Science, 2021, 39(1):89-96. doi: 10.3969/j.issn.1003-6563.2021.01.018
XIE J, ZHANG Q. Density-functional theory study of bonding properties at Ca sites of fluorapatite[J]. Guizhou Science, 2021, 39(1):89-96. doi: 10.3969/j.issn.1003-6563.2021.01.018
[3] 张晋霞, 牛福生. 响应曲面法优化赤铁矿絮凝体浮选行为研究[J]. 矿产综合利用, 2021(3):22-26.ZHANG J X, NIU F S. Optimization of flotation behavior of hematite flocs in sodium oleate system using response surface methodology[J]. Multipurpose Utilization of Mineral Resources, 2021(3):22-26. doi: 10.3969/j.issn.1000-6532.2021.03.004
ZHANG J X, NIU F S. Optimization of flotation behavior of hematite flocs in sodium oleate system using response surface methodology[J]. Multipurpose Utilization of Mineral Resources, 2021(3):22-26. doi: 10.3969/j.issn.1000-6532.2021.03.004
[4] 李淑菲, 李强. 白钨矿浮选研究现状[J]. 矿产综合利用, 2019(3):17-21.LI S F, LI Q. Current research situation of scheelite flotation[J]. Multipurpose Utilization of Mineral Resources, 2019(3):17-21. doi: 10.3969/j.issn.1000-6532.2019.03.004
LI S F, LI Q. Current research situation of scheelite flotation[J]. Multipurpose Utilization of Mineral Resources, 2019(3):17-21. doi: 10.3969/j.issn.1000-6532.2019.03.004
[5] 刘忠义, 马子龙, 刘杰, 等. 含云母方解石型萤石矿的浮选柱分选试验研究[J]. 矿产综合利用, 2019(2):60-64.LIU Z Y, MA Z L, LIU J, et al. Experimental research on flotation column separation of micaceous calcite-type fluorite[J]. Multipurpose Utilization of Mineral Resources, 2019(2):60-64. doi: 10.3969/j.issn.1000-6532.2019.02.012
LIU Z Y, MA Z L, LIU J, et al. Experimental research on flotation column separation of micaceous calcite-type fluorite[J]. Multipurpose Utilization of Mineral Resources, 2019(2):60-64. doi: 10.3969/j.issn.1000-6532.2019.02.012
[6] 崔瑞, 王旭, 魏骞, 等. 湖北某重晶石-萤石型矿综合利用研究[J]. 矿产综合利用, 2019(2):70-74.CUI R, WANG X, WEI Q, et al. Study on comprehensive utilization of a barite-fluorite ore in Hubei province[J]. Multipurpose Utilization of Mineral Resources, 2019(2):70-74. doi: 10.3969/j.issn.1000-6532.2019.02.014
CUI R, WANG X, WEI Q, et al. Study on comprehensive utilization of a barite-fluorite ore in Hubei province[J]. Multipurpose Utilization of Mineral Resources, 2019(2):70-74. doi: 10.3969/j.issn.1000-6532.2019.02.014
[7] 曾理, 姜小明. Gemini表面活性剂体系下钙质磷矿中白云石的可浮性研究[J]. 矿产综合利用, 2020(1):83-88.ZENG L, JIANG X M. Study on the floatability of dolomite in calcareous phosphate rock under Gemini surfactant system[J]. Multipurpose Utilization of Mineral Resources, 2020(1):83-88. doi: 10.3969/j.issn.1000-6532.2020.01.017
ZENG L, JIANG X M. Study on the floatability of dolomite in calcareous phosphate rock under Gemini surfactant system[J]. Multipurpose Utilization of Mineral Resources, 2020(1):83-88. doi: 10.3969/j.issn.1000-6532.2020.01.017
[8] 王杰, 张覃, 邱跃琴, 等. 方解石晶体结构及表面活性位点第一性原理[J]. 工程科学学报, 2017, 39(4):487-493.WANG J, ZHANG Q, QIU Y Q, et al. Calcite crystal structure and first principles of surface active sites[J]. Journal of Engineering Science, 2017, 39(4):487-493.
WANG J, ZHANG Q, QIU Y Q, et al. Calcite crystal structure and first principles of surface active sites[J]. Journal of Engineering Science, 2017, 39(4):487-493.
[9] 钱有军, 高莉. 萤石与方解石、重晶石等盐类矿物浮选分离现状[J]. 中国非金属矿工业导刊, 2014(4):18-21.QIAN Y J, GAO L. Current status of flotation separation of fluorite from calcite, barite and other salt minerals[J]. China Nonmetallic Mineral Industry Guide, 2014(4):18-21. doi: 10.3969/j.issn.1007-9386.2014.04.006
QIAN Y J, GAO L. Current status of flotation separation of fluorite from calcite, barite and other salt minerals[J]. China Nonmetallic Mineral Industry Guide, 2014(4):18-21. doi: 10.3969/j.issn.1007-9386.2014.04.006
[10] 屠建春. 难选氧化铅锌矿石铅浮选工艺研究[J]. 黄金, 2019, 40(9):52-55.TU J C. Research on lead flotation process of difficult-to-select oxidized lead-zinc ore[J]. Gold, 2019, 40(9):52-55. doi: 10.11792/hj20190912
TU J C. Research on lead flotation process of difficult-to-select oxidized lead-zinc ore[J]. Gold, 2019, 40(9):52-55. doi: 10.11792/hj20190912
[11] 从金瑶, 王维清, 林一明, 等. 油酸钠体系下钙离子活化石英浮选机理研究[J]. 非金属矿, 2018, 41(6):77-79.CONG J Y, WANG W Q, LIN Y M, et al. Study on flotation mechanism of calcium ion activated quartz under sodium oleate system[J]. Nonmetallic Mining, 2018, 41(6):77-79. doi: 10.3969/j.issn.1000-8098.2018.06.024
CONG J Y, WANG W Q, LIN Y M, et al. Study on flotation mechanism of calcium ion activated quartz under sodium oleate system[J]. Nonmetallic Mining, 2018, 41(6):77-79. doi: 10.3969/j.issn.1000-8098.2018.06.024
[12] 朱一民. 2019年浮选药剂的进展[J]. 矿产综合利用, 2020(5):1-17.ZHU Y M. Development of flotation reagent in 2019[J]. Multipurpose Utilization of Mineral Resources, 2020(5):1-17. doi: 10.3969/j.issn.1000-6532.2020.05.001
ZHU Y M. Development of flotation reagent in 2019[J]. Multipurpose Utilization of Mineral Resources, 2020(5):1-17. doi: 10.3969/j.issn.1000-6532.2020.05.001
[13] 朱一民. 2020年浮选药剂的进展[J]. 矿产综合利用, 2021(2):102-118.ZHU Y M. Development of flotation reagent in 2020[J]. Multipurpose Utilization of Mineral Resources, 2021(2):102-118. doi: 10.3969/j.issn.1000-6532.2021.02.019
ZHU Y M. Development of flotation reagent in 2020[J]. Multipurpose Utilization of Mineral Resources, 2021(2):102-118. doi: 10.3969/j.issn.1000-6532.2021.02.019
[14] 沈智慧, 张覃, 卯松, 等. 脂肪酸结构对胶磷矿表面润湿性的影响研究[J]. 矿产保护与利用, 2018(3):105-111.SHEN Z H, ZHANG Q, MAO S, et al. Study on the effect of fatty acid structure on the surface wettability of colophony[J]. Mineral Protection and Utilization, 2018(3):105-111.
SHEN Z H, ZHANG Q, MAO S, et al. Study on the effect of fatty acid structure on the surface wettability of colophony[J]. Mineral Protection and Utilization, 2018(3):105-111.
[15] 冯其明, 席振伟, 张国范, 等. 脂肪酸捕收剂浮选钛铁矿性能研究[J]. 金属矿山, 2009(5):46-49.FENG Q M, XI Z W, ZHANG G F, et al. Study on the flotation performance of ilmenite with fatty acid trap[J]. Metal Mining, 2009(5):46-49. doi: 10.3321/j.issn:1001-1250.2009.05.012
FENG Q M, XI Z W, ZHANG G F, et al. Study on the flotation performance of ilmenite with fatty acid trap[J]. Metal Mining, 2009(5):46-49. doi: 10.3321/j.issn:1001-1250.2009.05.012
[16] 张庆鹏, 刘润清, 曹学锋, 等. 脂肪酸类白钨矿捕收剂的结构性能关系研究[J]. 有色金属科学与工程, 2013, 4(5):85-90.ZHANG Q P, LIU R Q, CAO X F, et al. Structure-property relationship study of fatty acid-based scheelite traps[J]. Nonferrous Metal Science and Engineering, 2013, 4(5):85-90.
ZHANG Q P, LIU R Q, CAO X F, et al. Structure-property relationship study of fatty acid-based scheelite traps[J]. Nonferrous Metal Science and Engineering, 2013, 4(5):85-90.
[17] 杨耀辉. 白钨矿浮选过程中脂肪酸类捕收剂的混合效应[D]. 长沙: 中南大学, 2010.YANG Y H. Mixing effect of fatty acid-based traps during scheelite flotation[D]. Changsha: Central South University, 2010.
YANG Y H. Mixing effect of fatty acid-based traps during scheelite flotation[D]. Changsha: Central South University, 2010.
[18] Geneyton A, Foucaud Y, Filippov L O, et al. Synergistic adsorption of lanthanum ions and fatty acids for efficient rare-earth phosphate recovery: Surface analysis and ab initio molecular dynamics studies[J]. Applied Surface Science, 2020, 526:146725. doi: 10.1016/j.apsusc.2020.146725
[19] 卢绿荣, 陈建华, 李玉琼. 硫化矿浮选捕收剂分子结构与性能的电子态密度研究[J]. 中国有色金属学报, 2018, 28(7):1482-1490.LU L R, CHEN J H, LI Y Q. Electronic density of states study on the molecular structure and performance of sulfide ore flotation traps[J]. Chinese Journal of Nonferrous Metals, 2018, 28(7):1482-1490.
LU L R, CHEN J H, LI Y Q. Electronic density of states study on the molecular structure and performance of sulfide ore flotation traps[J]. Chinese Journal of Nonferrous Metals, 2018, 28(7):1482-1490.
[20] 王贤晨, 张覃, 陈建华, 等. 氟磷灰石与石英表面电子性质及胺类捕收剂吸附作用研究[J]. 贵州大学学报( 自然科学版), 2017, 34(6):21-28.WANG X C, ZHANG Q, CHEN J H, et al. Study on the electronic properties of fluorapatite and quartz surfaces and adsorption of amine traps[J]. Journal of Guizhou University ( Natural Science Edition ), 2017, 34(6):21-28.
WANG X C, ZHANG Q, CHEN J H, et al. Study on the electronic properties of fluorapatite and quartz surfaces and adsorption of amine traps[J]. Journal of Guizhou University ( Natural Science Edition ), 2017, 34(6):21-28.
[21] 柴汝宽, 刘月田, 杨莉, 等. 乙酸在方解石表面吸附的密度泛函研究[J]. 中南大学学报(自然科学版), 2019, 50(5):1252-1262.CHAI R K, LIU Y T, YANG L, et al. Density functional study of acetic acid adsorption on calcite surface[J]. Journal of Central South University (Natural Science Edition), 2019, 50(5):1252-1262.
CHAI R K, LIU Y T, YANG L, et al. Density functional study of acetic acid adsorption on calcite surface[J]. Journal of Central South University (Natural Science Edition), 2019, 50(5):1252-1262.
[22] Gao Z Y, Xie L, Cui X, et al. Probing anisotropic surface properties and surface forces of fluorite crystals[J]. Langmuir, 2018, 34(7):2511-2521. doi: 10.1021/acs.langmuir.7b04165
[23] Xie J, Li X H, Mao S, et al. Effects of structure of fatty acid collectors on the adsorption of fluorapatite (0 0 1) surface: A first-principles calculations[J]. Applied Surface Science, 2018, 444:699-709. doi: 10.1016/j.apsusc.2018.03.105
[24] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988.WANG D Z, HU Y H. Chemistry of flotation solutions [M]. Changsha: Hunan Science and Technology Press, 1988.
WANG D Z, HU Y H. Chemistry of flotation solutions [M]. Changsha: Hunan Science and Technology Press, 1988.
[25] 朱建光. 浮选药剂[M]. 北京: 冶金工业出版社, 1993.ZHU J G. Flotation pharmacy [M]. Beijing: Metallurgical Industry Press, 1993.
ZHU J G. Flotation pharmacy [M]. Beijing: Metallurgical Industry Press, 1993.
[26] Foucaud Y, Lebègue S, Filippov L O, et al. Molecular insight into fatty acid adsorption on bare and hydrated (111) fluorite surface[J]. The Journal of Physical Chemistry B, 2018, 122(51):12403-12410. doi: 10.1021/acs.jpcb.8b08969
[27] 王淳纯, 覃小丽, 阚建全, 等. 量子化学计算法比较不同脂肪酸分子的反应活性位点[J]. 食品科学, 2021, 42(8):74-80.WANG C C, QIN X L, KAN J Q, et al. Comparison of reactive sites of different fatty acid molecules by quantum chemical calculations[J]. Food Science, 2021, 42(8):74-80. doi: 10.7506/spkx1002-6630-20200316-253
WANG C C, QIN X L, KAN J Q, et al. Comparison of reactive sites of different fatty acid molecules by quantum chemical calculations[J]. Food Science, 2021, 42(8):74-80. doi: 10.7506/spkx1002-6630-20200316-253
[28] 王淀佐. 浮选剂作用原理及应用[M]. 北京: 冶金工业出版社, 1981.WANG D Z. Principles of flotation agent action and application [M]. Beijing: Metallurgical Industry Press, 1981.
WANG D Z. Principles of flotation agent action and application [M]. Beijing: Metallurgical Industry Press, 1981.
-



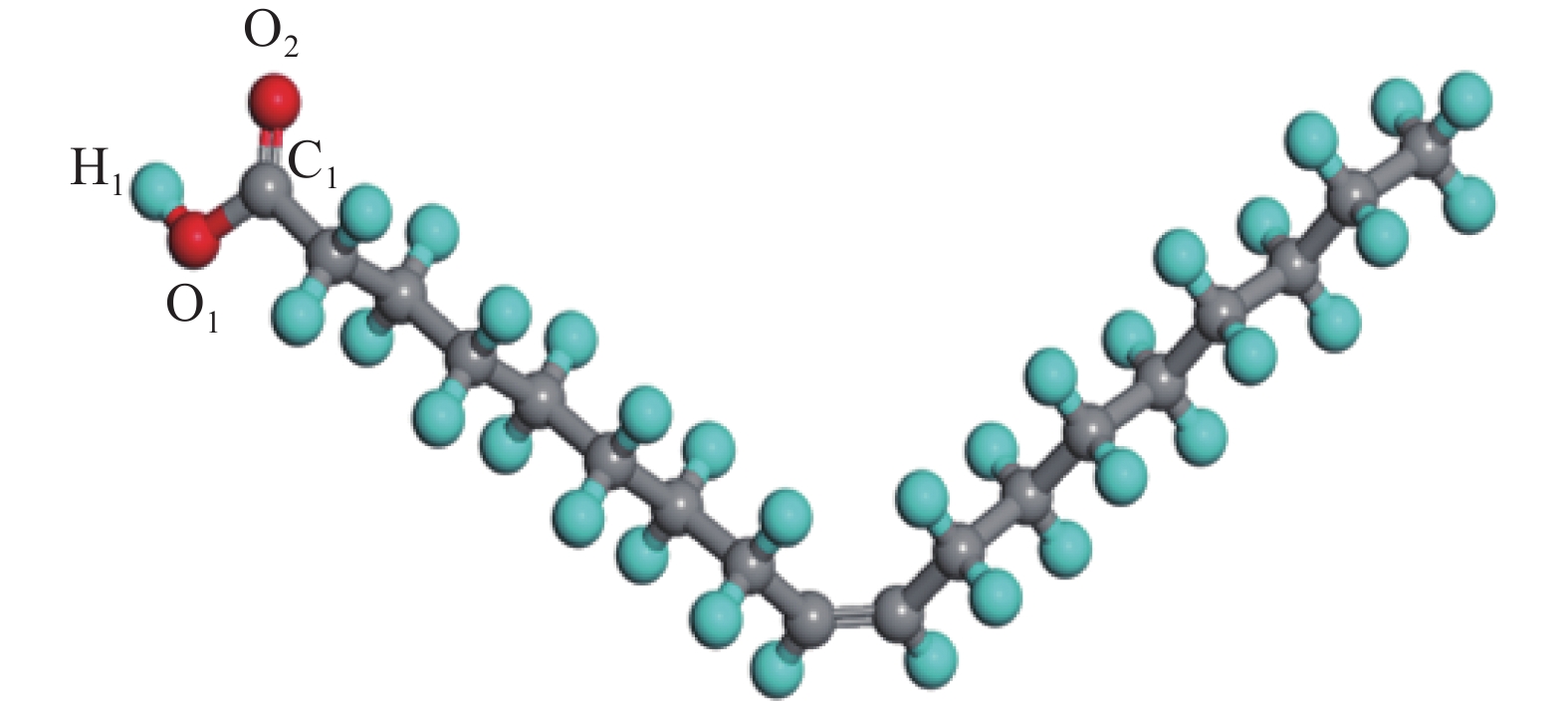
 下载:
下载: