The Effect of Dry-Ashing Method on Copper Isotopic Analysis of Soil Samples with Organic Matter
-
摘要: 分析土壤样品的铜同位素时,其中有机质会对化学纯化流程以及测试过程产生严重的干扰。因此,在不改变样品铜同位素组成的前提下,完全去除土壤中的有机质对于获取高精度的铜同位素数据至关重要。干法灰化是一种快速、有效的有机质处理方法,并且能够减少氧化性试剂的使用。但是该流程可能会对挥发性元素(如铜)的组成产生影响,因此需要在使用前进行条件实验探究。本文采用干法灰化流程对含有机质的土壤样品进行有机质处理,同时使用高压湿法消解对相同的样品进行处理,并分别在纯化后用MC-ICP-MS测量铜同位素组成,通过两种处理方式测量结果的对比,探求干法灰化法对土壤样品铜同位素组成的影响程度。结果表明:高压湿法消解流程能够获得较为可靠的铜同位素组成数据;干法灰化流程使铜同位素组成的测量值显著偏离真实值,δ65Cu最多可偏差3.46‰。这是因为样品中铜的挥发丢失导致了铜同位素组成发生分馏,并且其影响程度受到了多种因素控制,如样品性质和灰化温度等。因此,实验结果更推荐使用湿法消解对土壤样品进行处理。Abstract:
BACKGROUNDWhen analyzing copper isotopes in soil samples, organic matter can cause serious interference on chemical purification and analysis processes. Therefore, the complete removal of organic matter from the soil is critical to obtain high-precision copper isotope data without changing the copper isotope composition of the sample. OBJECTIVESTo carry out the condition experiments before using the dry-ashing method, because the method may influence the compositions of volatile elements, such as Cu, in soil samples. METHODSSoil samples containing organic matter were treated by dry-ashing and then digested by the high-pressure wet digestion method. The copper isotope composition was measured by Multi-collector Inductively Coupled Plasma-Mass Spectrometry after purification. Through the comparison of the results of the two treatment methods, the extent of influence of the dry-ashing method on the copper isotopic composition of soil samples was investigated. RESULTSThe results demonstrate that the wet-digestion method can provide more precise results, while the dry-ashing method can significantly fractionate Cu isotopes of soil samples. The deviation of δ 65Cu values varies from -0.61‰ to 3.46‰ for the dry-ashing method, which was caused by volatilization loss of Cu. Moreover, the degree of influence is controlled by a few factors such as sample nature and ashing temperature. CONCLUSIONSThe dry-ashing method is not an appropriate method for soil sample digestion. The wet-digestion method is a better choice to prepare samples for Cu isotopic analysis. -

-
表 1 铜同位素化学纯化流程
Table 1. Chemical purification procedure for Cu isotopes
所用试剂 试剂体积
(mL)操作步骤 0.5 mol/L硝酸 30 清洗树脂 二次去离子水 30 清洗树脂 6 mol/L盐酸+0.001%过氧化氢 8 平衡树脂 6 mol/L盐酸+0.001%过氧化氢 1 上样 6 mol/L盐酸+0.001%过氧化氢 4 淋洗基质 6 mol/L盐酸+0.001%过氧化氢 1 接取基质 6 mol/L盐酸+0.001%过氧化氢 26 接取铜 6 mol/L盐酸+0.001%过氧化氢 1 接取基质 0.5 mol/L硝酸 10 清洗树脂 二次去离子水 10 清洗树脂 表 2 国际地球化学标准物质的铜同位素组成测量结果
Table 2. Copper isotopic compositions of international geochemical standard reference materials
样品编号 δ65Cu①
(‰)2SD②
(‰)n 来源文献 0.13 0.03 3 本研究 AGV-2 0.10 0.10 8 Moynier等(2010)[40] 0.11 0.04 3 Moeller等(2012)[39] 0.07 0.04 3 本研究 BHVO-2 0.13 0.06 18 Liu等(2014)[26] 0.10 0.05 9 Huang等(2017)[4] 0.07 0.06 - Dekov等(2013)[41] 0.27 0.05 3 本研究 BCR-2 0.22 0.04 2 Liu等(2014)[26] 0.20 0.04 3 Huang等(2017)[4] 注:① $ {\mathit{\delta }^{{\rm{65}}}}{\rm{Cu = }}\left[ {\frac{{{{\left( {^{{\rm{65}}}{\rm{Cu}}{{\rm{/}}^{{\rm{63}}}}{\rm{Cu}}} \right)}_{{\rm{样品}}}}}}{{{{\left( {^{{\rm{65}}}{\rm{Cu}}{{\rm{/}}^{{\rm{63}}}}{\rm{Cu}}} \right)}_{{\rm{NIST}}\;{\rm{SRM976}}}}}}{\rm{ - 1}}} \right]{\rm{ \times 1000‰}}$ 。
② 2SD:一份溶液测量n次的标准偏差的2倍。表 3 干法灰化和高压湿法消解处理土壤样品的铜同位素组成测量结果
Table 3. Copper isotopic compositions of soil samples pretreated with dry-ashing and high-pressure wet digestion methods
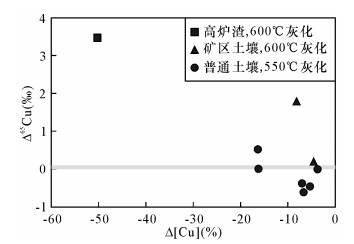
样品名称① 干法灰化 样品类型 Δ[Cu]②(%) Δ65Cu③ (‰) δ65Cu④ (‰) 2SD⑤ (‰) n USTC-1 (Ⅱ) 高炉渣 -50.2 3.46 4.75 0.38 3 USTC-2 (Ⅱ) 矿区土壤 -4.7 0.16 0.15 0.05 3 USTC-3 (Ⅱ) 矿区土壤 -8.3 1.74 1.77 0.16 3 Repeat⑥ - -6.6 - 1.81 0.01 3 USTC-4 (Ⅰ) 普通土壤 -5.5 -0.45 0.47 0.01 3 USTC-5 (Ⅰ) 普通土壤 -16.3 0.02 0.14 0.04 3 USTC-6 (Ⅰ) 普通土壤 -7.1 -0.37 0.35 0.03 3 USTC-7 (Ⅰ) 普通土壤 -16.4 0.53 0.66 0.06 3 USTC-8 (Ⅰ) 普通土壤 -3.8 0.00 0.11 0.07 3 USTC-9 (Ⅰ) 普通土壤 -6.8 -0.61 0.07 0.04 3 样品名称① 高压湿法消解 样品类型 Δ[Cu]②(%) Δ65Cu③ (‰) δ65Cu④ (‰) 2SD⑤ (‰) n USTC-1 (Ⅲ) 高炉渣 2.6 - 1.29 0.08 3 USTC-2 (Ⅲ) 矿区土壤 -3.4 - -0.01 0.04 3 USTC-3 (Ⅲ) 矿区土壤 3.2 - -0.07 0.01 3 USTC-4 (Ⅲ) 普通土壤 -2.2 - 0.92 0.03 3 USTC-5 (Ⅲ) 普通土壤 -4.3 - 0.12 0.04 3 USTC-6 (Ⅲ) 普通土壤 1.7 - 0.72 0.04 3 USTC-7 (Ⅲ) 普通土壤 -3.0 - 0.14 0.02 3 Repeat⑥ - 2.2 - 0.16 0.04 3 USTC-8 (Ⅲ) 普通土壤 1.9 - 0.10 0.03 3 USTC-9 (Ⅲ) 普通土壤 2.2 - 0.68 0.02 3 注:①样品名称中后缀(Ⅰ)代表 550℃灰化的样品,(Ⅱ)代表 600℃灰化的样品,(Ⅲ)代表使用压力消解罐进行高压湿法消解。
②样品经过化学流程处理后的铜最终剩余量和铜初始称样量的相对偏差,Δ[Cu]=([Cu]剩余/[Cu]初始-1)×100%。
③ Δ65Cu=δ65Cu干法灰化-δ65Cu高压湿法消解。
④$ {\mathit{\delta }^{{\rm{65}}}}{\rm{Cu = }}\left[ {\frac{{{{\left( {^{{\rm{65}}}{\rm{Cu}}{{\rm{/}}^{{\rm{63}}}}{\rm{Cu}}} \right)}_{{\rm{样品}}}}}}{{{{\left( {^{{\rm{65}}}{\rm{Cu}}{{\rm{/}}^{{\rm{63}}}}{\rm{Cu}}} \right)}_{{\rm{NIST}}\;{\rm{SRM976}}}}}}{\rm{ - 1}}} \right]{\rm{ \times 1000‰}}$ 。
⑤ 2SD:一份溶液测量n次的标准偏差的2倍。
⑥ Repeat:同一样品分别进行化学纯化流程和测试。 -
[1] Mathur R, Titley S, Barra F, et al.Exploration potential of Cu isotope fractionation in porphyry copper deposits[J].Journal of Geochemical Exploration, 2009, 102:1-6. doi: 10.1016/j.gexplo.2008.09.004
[2] Asael D, Matthews A, Bar-Matthews M, et al.Copper isotope fractionation in sedimentary copper mineralization (Timna Valley, Israel)[J].Chemical Geology, 2007, 243:238-254. doi: 10.1016/j.chemgeo.2007.06.007
[3] Larson P B, Maher K, Ramos F C, et al.Copper isotope ratios in magmatic and hydrothermal ore-forming environments[J].Chemical Geology, 2003, 201:337-350. doi: 10.1016/j.chemgeo.2003.08.006
[4] Huang J, Huang F, Wang Z, et al.Copper isotope frac-tionation during partial melting and melt percolation in the upper mantle:Evidence from Massif peridotites in Ivrea-Verbano Zone, Italian Alps[J].Geochimica et Cosmochimica Acta, 2017, 211:48-63. doi: 10.1016/j.gca.2017.05.007
[5] Li W Q, Jackson S E, Pearson N J, et al.The Cu isotopic signature of granites from the Lachlan fold belt, SE Australia[J].Chemical Geology, 2009, 258:38-49. doi: 10.1016/j.chemgeo.2008.06.047
[6] Bigalke M, Weyer S, Wilcke W.Stable Cu isotope frac-tionation in soils during oxic weathering and podzolization[J].Geochimica et Cosmochimica Acta, 2011, 75:3119-3134. doi: 10.1016/j.gca.2011.03.005
[7] Vance D, Archer C, Bermin J, et al.The copper isotope geochemistry of rivers and the oceans[J].Earth and Planetary Science Letters, 2008, 274:204-213. doi: 10.1016/j.epsl.2008.07.026
[8] Gonzalez R O, Strekopytov S, Amato F, et al.New insights from zinc and copper isotopic compositions into the sources of atmospheric particulate matter from two major European cities[J].Environmental Science & Technology, 2016, 50:9816-9824. http://www.ncbi.nlm.nih.gov/pubmed/27508898
[9] Thapalia A, Borrok D M, van Metre P C, et al.Zn and Cu isotopes as tracers of anthropogenic contamination in a sediment core from an urban lake[J].Environmental Science & Technology, 2010, 44:1544-1550. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0215604902
[10] Télouk P, Puisieux A, Fujii T, et al.Copper isotope effect in serum of cancer patients:A pilot study[J].Metallomics, 2015, 7:299-308. doi: 10.1039/C4MT00269E
[11] Navarrete J U, Borrok D M, Viveros M, et al.Copper iso-tope fractionation during surface adsorption and intracellular incorporation by bacteria[J].Geochimica et Cosmochimica Acta, 2011, 75:784-799. doi: 10.1016/j.gca.2010.11.011
[12] Mathur R, Titley S, Hart G, et al.The history of the Uni-ted States cent revealed through copper isotope fractionation[J].Journal of Archaeological Science, 2009, 36:430-433. doi: 10.1016/j.jas.2008.09.029
[13] Moynier F, Albarède F, Herzog G.Isotopic composition of zinc, copper, and iron in Lunar samples[J].Geochimica et Cosmochimica Acta, 2006, 70:6103-6117. doi: 10.1016/j.gca.2006.02.030
[14] Luck J M, Othman D B, Albarède F.Zn and Cu isotopic variations in chondrites and iron meteorites:Early Solar nebula reservoirs and parent-body processes[J].Geochimica et Cosmochimica Acta, 2005, 69:5351-5363. doi: 10.1016/j.gca.2005.06.018
[15] Vance D, Matthews A, Keech A, et al.The behaviour of Cu and Zn isotopes during soil development:Controls on the dissolved load of rivers[J].Chemical Geology, 2016, 445:36-53. doi: 10.1016/j.chemgeo.2016.06.002
[16] Liu S A, Teng F Z, Li S, et al.Copper and iron isotope fractionation during weathering and pedogenesis:Insights from saprolite profiles[J].Geochimica et Cosmochimica Acta, 2014, 146:59-75. doi: 10.1016/j.gca.2014.09.040
[17] Grybos M, Davranche M, Gruau G, et al.Is trace metal release in wetland soils controlled by organic matter mobility or Fe-oxyhydroxides reduction?[J].Journal of Colloid and Interface Science, 2007, 314:490-501. doi: 10.1016/j.jcis.2007.04.062
[18] Maréchal C N, Télouk P, Albarède F.Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry[J].Chemical Geology, 1999, 156:251-273. doi: 10.1016/S0009-2541(98)00191-0
[19] Mason T F, Weiss D J, Horstwood M, et al.High-precision Cu and Zn isotope analysis by plasma source mass spectrometry.Part 1.Spectral interferences and their correction[J].Journal of Analytical Atomic Spectrometry, 2004, 19:209-217. doi: 10.1039/b306958c
[20] Mason T F, Weiss D J, Horstwood M, et al.High-precision Cu and Zn isotope analysis by plasma source mass spectrometry.Part 2.Correcting for mass discrimination effects[J].Journal of Analytical Atomic Spectrometry, 2004, 19:218-226. doi: 10.1039/b306953b
[21] Bermin J, Vance D, Archer C, et al.The determination of the isotopic composition of Cu and Zn in seawater[J].Chemical Geology, 2006, 226:280-297. doi: 10.1016/j.chemgeo.2005.09.025
[22] Peel K, Weiss D, Chapman J, et al.A simple combined sample-standard bracketing and inter-element correction procedure for accurate mass bias correction and precise Zn and Cu isotope ratio measurements[J].Journal of Analytical Atomic Spectrometry, 2008, 23:103-110. doi: 10.1039/B710977F
[23] Sossi P A, Halverson G P, Nebel O, et al.Combined se-paration of Cu, Fe and Zn from rock matrices and improved analytical protocols for stable isotope determination[J].Geostandards and Geoanalytical Research, 2015, 39:129-149. doi: 10.1111/ggr.2015.39.issue-2
[24] Zhu X, O'nions R, Guo Y, et al.Determination of natural Cu-isotope variation by plasma-source mass spectrometry:Implications for use as geochemical tracers[J].Chemical Geology, 2000, 163:139-149. doi: 10.1016/S0009-2541(99)00076-5
[25] Larner F, Rehkämper M, Coles B J, et al.A new separation procedure for Cu prior to stable isotope analysis by MC-ICP-MS[J].Journal of Analytical Atomic Spectrometry, 2011, 26:1627-1632. doi: 10.1039/c1ja10067j
[26] Liu S A, Li D, Li S, et al.High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS[J].Journal of Analytical Atomic Spectrometry, 2014, 29:122-133. doi: 10.1039/C3JA50232E
[27] Albarède F, Telouk P, Blichert-Toft J, et al.Precise and accurate isotopic measurements using multiple-collector ICPMS[J].Geochimica et Cosmochimica Acta, 2004, 68:2725-2744. doi: 10.1016/j.gca.2003.11.024
[28] Yang L.Accurate and precise determination of isotopic ratios by MC-ICP-MS:A review[J].Mass Spectrometry Reviews, 2009, 28:990-1011. doi: 10.1002/mas.v28:6
[29] Douthitt C B.The evolution and applications of multi-collector ICPMS (MC-ICPMS)[J].Analytical & Bioanalytical Chemistry, 2008, 390:437-440. http://www.ncbi.nlm.nih.gov/pubmed/17938893
[30] Fontaine G H, Hattendorf B, Bourdon B, et al.Effects of operating conditions and matrix on mass bias in MC-ICPMS[J].Journal of Analytical Atomic Spectrometry, 2009, 24:637-648. doi: 10.1039/b816948a
[31] Barling J, Weis D.An isotopic perspective on mass bias and matrix effects in multi-collector inductively-coupled-plasma mass spectrometry[J].Journal of Analytical Atomic Spectrometry, 2012, 27:653-662. doi: 10.1039/c2ja10382f
[32] 李世珍, 朱祥坤, 吴龙华, 等.干法灰化和湿法消解植物样品的铜锌铁同位素测定对比研究[J].地球学报, 2011, 32(6):116-122. http://d.old.wanfangdata.com.cn/Periodical/dqxb201106014
Li S Z, Zhu X K, Wu L H, et al.A comparative study of plant sample preperation by bry ashing and wet digestion for isotopic determination of Cu, Zn and Fe[J].Acta Geoscientica Sinica, 2011, 32(6):116-122. http://d.old.wanfangdata.com.cn/Periodical/dqxb201106014
[33] Jenkins E.Phytolith taphonomy:A comparison of dry ashing and acid extraction on the breakdown of conjoined phytoliths formed in Triticum Durum[J].Journal of Archaeological Science, 2009, 36:2402-2407. doi: 10.1016/j.jas.2009.06.028
[34] Sahrawat K, Ravi K G, Rao J.Evaluation of triacid and dry ashing procedures for determining potassium, calcium, magnesium, iron, zinc, manganese, and copper in plant materials[J].Communications in Soil Science and Plant Analysis, 2002, 33:95-102. doi: 10.1081/CSS-120002380
[35] Enders A, Lehmann J.Comparison of wet-digestion and dry-ashing methods for total elemental analysis of biochar[J].Communications in Soil Science and Plant Analysis, 2012, 43:1042-1052. doi: 10.1080/00103624.2012.656167
[36] 乔爱香, 曹磊, 江冶, 等.干法灰化和微波消解-电感耦合等离子体发射光谱法测定植物样品中22个主次量元素[J].岩矿测试, 2010, 29(1):29-33. doi: 10.3969/j.issn.0254-5357.2010.01.007 http://www.ykcs.ac.cn/article/id/ykcs_20100107
Qiao A X, Cao L, Jiang Y, et al.Inductively coupled plasma-atomic emission spectrometric determination of 22 major and minor elements in plant samples with sample pretreatment methods of dry ashing and microwave digestion[J].Rock and Mineral Analysis, 2010, 29(1):29-33. doi: 10.3969/j.issn.0254-5357.2010.01.007 http://www.ykcs.ac.cn/article/id/ykcs_20100107
[37] Lodders K.Solar system abundances and condensation temperatures of the elements[J].Astrophysical Journal, 2003, 591:1220-1247. doi: 10.1086/apj.2003.591.issue-2
[38] 侯振辉, 王晨香.电感耦合等离子体质谱法测定地质样品中35种微量元素[J].中国科学技术大学学报, 2007, 37(8):940-944. doi: 10.3969/j.issn.0253-2778.2007.08.019
Hou Z H, Wang C X.Determination of 35 trace elements in geological samples by inductively coupled plasma mass spectrometry[J].Jouenal of University of Science and Technology of China, 2007, 37(8):940-944. doi: 10.3969/j.issn.0253-2778.2007.08.019
[39] Moeller K, Schoenberg R, Pedersen R B, et al.Calibra-tion of the new certified reference materials ERM-AE633 and ERM-AE647 for copper and IRMM-3702 for zinc isotope amount ratio determinations[J]. Geostandards and Geoanalytical Research, 2012, 36:177-199. doi: 10.1111/ggr.2012.36.issue-2
[40] Moynier F, Koeberl C, Beck P, et al.Isotopic fractiona-tion of Cu in Tektites[J].Geochimica et Cosmochimica Acta, 2010, 74:799-807. doi: 10.1016/j.gca.2009.10.012
[41] Dekov V M, Rouxel O, Asael D, et al.Native Cu from the oceanic crust:Isotopic insights into native metal origin[J].Chemical Geology, 2013, 359:136-149. doi: 10.1016/j.chemgeo.2013.10.001
[42] Young E D, Galy A, Nagahara H.Kinetic and equilibri-um mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical significance[J].Geochimica et Cosmochimica Acta, 2002, 66:1095-1104. doi: 10.1016/S0016-7037(01)00832-8
[43] Bowen H.The determination of copper and zinc in biolo-gical material by activation analysis[J].The International Journal of Applied Radiation and Isotopes, 1959, 4:214-220. doi: 10.1016/0020-708X(59)90196-6
-



 下载:
下载: