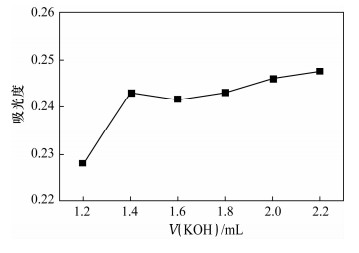
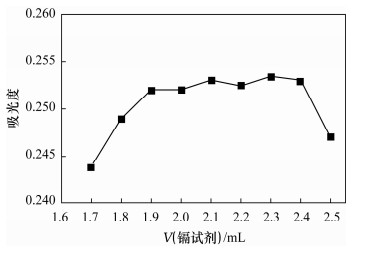
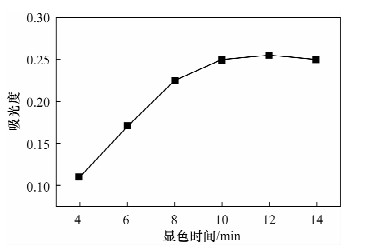
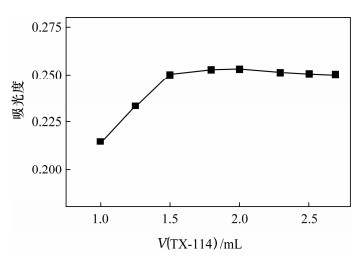
Spectrophotometric Determination of Cd(Ⅱ) in Environmental Water Samples with Cadion in the Presence of Triton X-114 Surface Active Agent
-
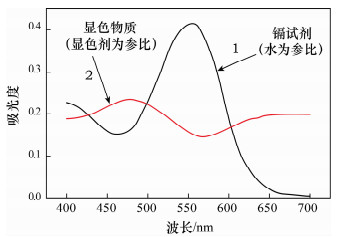
摘要: 及时监测环境水中的镉含量是预防和治理镉污染的前提,采用分光光度法测定镉具有简便、迅速、易普及等特点,然而较低的灵敏度限制了该方法的应用。本文通过选用表面活性剂Triton X-114为增敏剂,有效地提高了镉试剂分光光度法测定镉离子的灵敏度。研究结果表明,在KOH溶液中,Triton X-114存在下,镉试剂与镉离子生成橙红色络合物,显色物质最大吸收峰为470 nm,镉离子含量在0~10 μg/25 mL范围内符合比尔定律,表观摩尔吸光系数达到1.25×105 L/(mol·cm),检测限低至5.0 μg/L。与Triton X-100以及其他表面活性剂相比,Triton X-114的应用能显著提高镉试剂光度法测定镉的吸光度,表观摩尔吸光系数最高可增加49%,检测限也能够降低近50%,并且分析方法简便迅速,易普及,可应用于环境水以及工业废水中微量或痕量镉的直接测定。Abstract: It is vital to monitor the cadmium (Cd) content in environmental water over time, as it is a prerequisite to prevent and control Cd contamination. Spectrophotometric determination of Cd was simple, rapid and well established. However, low sensitivity limits the application of Spectrophotometry to determine Cd content in environmental water samples. The sensitivity of Cd can be significantly improved for Spectrophotometry by using the surface active agent Triton X-114. In a KOH medium, Cd ions react with cadion to form orange complex. In tests, the maximum absorption and molar absorptivity for the orange complex were 470 nm and 1.25×105L/(mol·cm), respectively. Beer′s law was obeyed for 0-10 μg of Cd in 25 mL of solution. The detection limit was 5.0 μg/L. Compared with Triton X-100 and other surface active agents, Triton X-114 can increase maximum of 49% to the molar absorption coefficient and reduced about 50% of the detection limit. This method is simple, fast and suitable to determine directly major or trace Cd in environmental water and industrial wastewater.
-
Key words:
- environmental water samples /
- cadmium /
- cadion /
- Triton X-114 /
- surface active agent /
- spectrophotometry
-

-
表 1 不同方法的线性范围、灵敏度和相对标准偏差
Table 1. Linear ranges,sensitivities and relative standard deviations of different methods
测试方法 线性范围ρ(Cd)/
(μg·25 mL-1)ε470/
(L·mol-1·cm-1)RSD/%
(n=5)检测限/
(μg·L-1)镉试剂-TX-114 0~10 1.25×105 2.205 5.0 镉试剂-TX-100 0~10 8.17×104 3.443 7.5 表 2 环境水样中Cd的测定结果
Table 2. Analytical results of Cd in environment water samples
实际水样 ρ(Cd)/(μg·mL-1) 回收率/% FAAS法测定总量ρ
(Cd)/(μg·mL-1)本底值 加入量 测定总量 湖水 未检出 0.050 0.048 95.5 0.048 工业废水 0.210 0.200 0.410 100.0 0.415 -
[1] 国家环境保护总局.水和废水监测分析方法[M].北京:中国环境科学出版社,2002: 323.
[2] 黄秋婵,韦友欢,黎晓峰.镉对人体健康的危害效应及其机理研究进展[J].安徽农业科学,2007,35(9): 2528-2531. http://www.cnki.com.cn/Article/CJFDTOTAL-AHNY200709007.htm
[3] 李昌明,魏祖安,李文华,韦桂水,梅冰,蒋影.石墨炉原子吸收法快速连续监测柳江水中镉[J].中国卫生检验杂志,2012,22(6): 1298-1300. http://www.cnki.com.cn/Article/CJFDTOTAL-ZWJZ201206032.htm
[4] 朱振科,陈建国,金献忠,陈少鸿,葛宣宁,魏丹毅.可溶性滤膜分离富集电感耦合等离子体原子发射光谱法对水中痕量镉铜铅与锌的同时测定[J].分析测试学报,2010,29(6): 599-602. http://www.cnki.com.cn/Article/CJFDTOTAL-TEST201006015.htm
[5] 王锋.示波极谱法测定饮料中铅和镉[J].理化检验: 化学分册,2005,41(3): 204-205. http://www.cnki.com.cn/Article/CJFDTOTAL-LHJH20050300K.htm
[6] 倪超,朱超云,宋伟.多壁碳纳米管铋膜修饰电极示差脉冲溶出伏安法测定水中镉和铅[J].光谱实验室,2011,28(4): 1943-1947. http://www.cnki.com.cn/Article/CJFDTOTAL-GPSS201104092.htm
[7] 周丽萍,李中玺.王水提取-电感耦合等离子体质谱法同时测定地质样品中微量银、镉、铋[J].分析试验室,2005,24(9): 20-25. http://www.cnki.com.cn/Article/CJFDTOTAL-FXSY200509005.htm
[8] 陈明丽,付海阔,孟皓,王建华.离子液体双水相萃取蒸气发生原子荧光法测定痕量镉[J].分析化学,2010,38(9): 1299-1304. http://www.cnki.com.cn/Article/CJFDTOTAL-FXHX201009014.htm
[9] 于秀兰,田松涛,孟凡金.三氮烯类试剂在光度分析中的应用进展[J].岩矿测试,2011,30(2): 131-137. http://www.cnki.com.cn/Article/CJFDTOTAL-SDHW200802014.htm
[10] 魏复盛,滕恩江,李前荣,沈乃葵.新镉试剂双波长分光光度法测定ppb级痕量镉[J].分析化学,1985,13(10): 762. http://www.cnki.com.cn/Article/CJFDTOTAL-FXHX198510011.htm
[11] 颜莎,满瑞林,彭天兰,乔亮杰.镉试剂正负峰加和法测定水中镉[J].分析试验室,2009,28(11): 68-70. doi: 10.3969/j.issn.1000-0720.2009.11.018
[12] 麻威武,张春牛,郑云法.1-(4-硝基苯基)-3-(5,6-二甲基-1,2,4三氮唑)-三氮烯的合成及与镉的显色反应[J].岩矿测试,2007,26(6): 469-471.
[13] 樊学忠,刘志平,聂天明.新显色剂2-氯-5-羧基苯重氮氨基偶氮苯的合成及与镉显色反应的研究[J].分析试验室,1994,13(6): 39.
[14] Hsu C G, Hu C S, Jing J H. Spectrophotometric deter-mination of cadmium with cadion [J].Talanta,1980,27(1): 676-678.
[15] 徐钟隽,胡昭圣,金基红.用镉试剂光度测定工业废水中微量镉[J].华东师范大学学报: 自然科学版,1979(1): 74-80. http://www.cnki.com.cn/Article/CJFDTOTAL-HDSZ197901013.htm
[16] 虞精明,谢勤美,吴爱贞.环境水样中表面活性剂的光度分析进展[J].中国卫生检验杂志,2007,17(7):1330-1332. http://www.cnki.com.cn/Article/CJFDTOTAL-ZWJZ200707086.htm
-



 下载:
下载: